Preterm Birth and PROM Testing Market Size - Global Industry, Share, Analysis, Trends and Forecast 2024 - 2032
Published :
Report ID:
Pages :
Format :
Preterm Birth and PROM Testing Market Size - Global Industry, Share, Analysis, Trends and Forecast 2024 - 2032
Report Coverage
- Industry Dynamics
- Market Size and Forecast Data
- Segment Analysis
- Competitive Landscape
- Regional Analysis with a Niche Focus on Country-Level Data
- High Level Analysis - Porter's, PESTEL, Value Chain, etc.
- Company Profiles of Key Players
- Option to Customize the Report As Per Your Specific Need
Request Sample Report
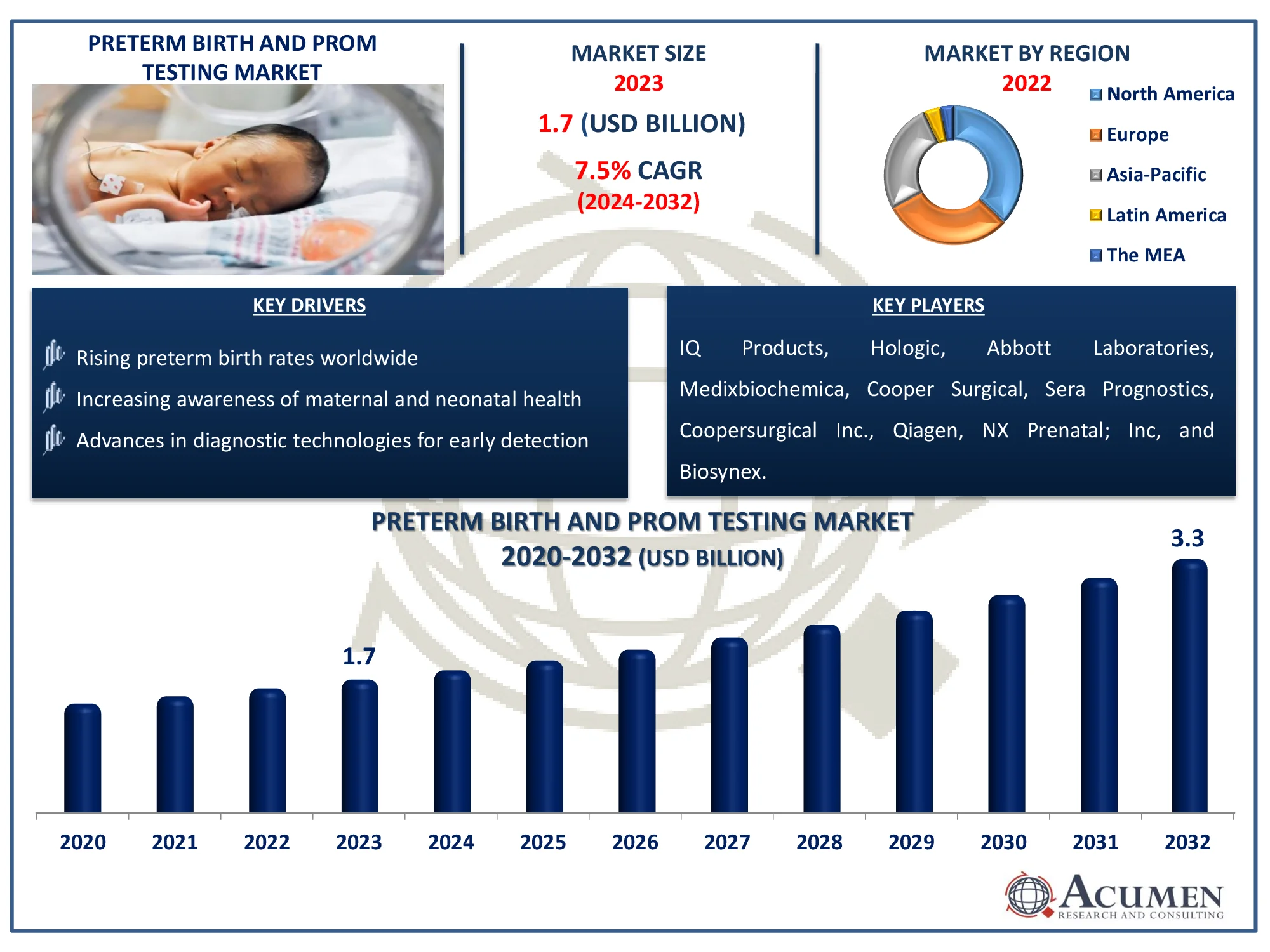
The Global Preterm Birth And PROM Testing Market Size accounted for USD 1.7 Billion in 2023 and is estimated to achieve a market size of USD 3.3 Billion by 2032 growing at a CAGR of 7.5% from 2024 to 2032.
Preterm Birth And PROM Testing Market Highlights
- The global preterm birth and PROM testing market is projected to reach USD 3.3 billion by 2032, with a CAGR of 7.5% from 2024 to 2032
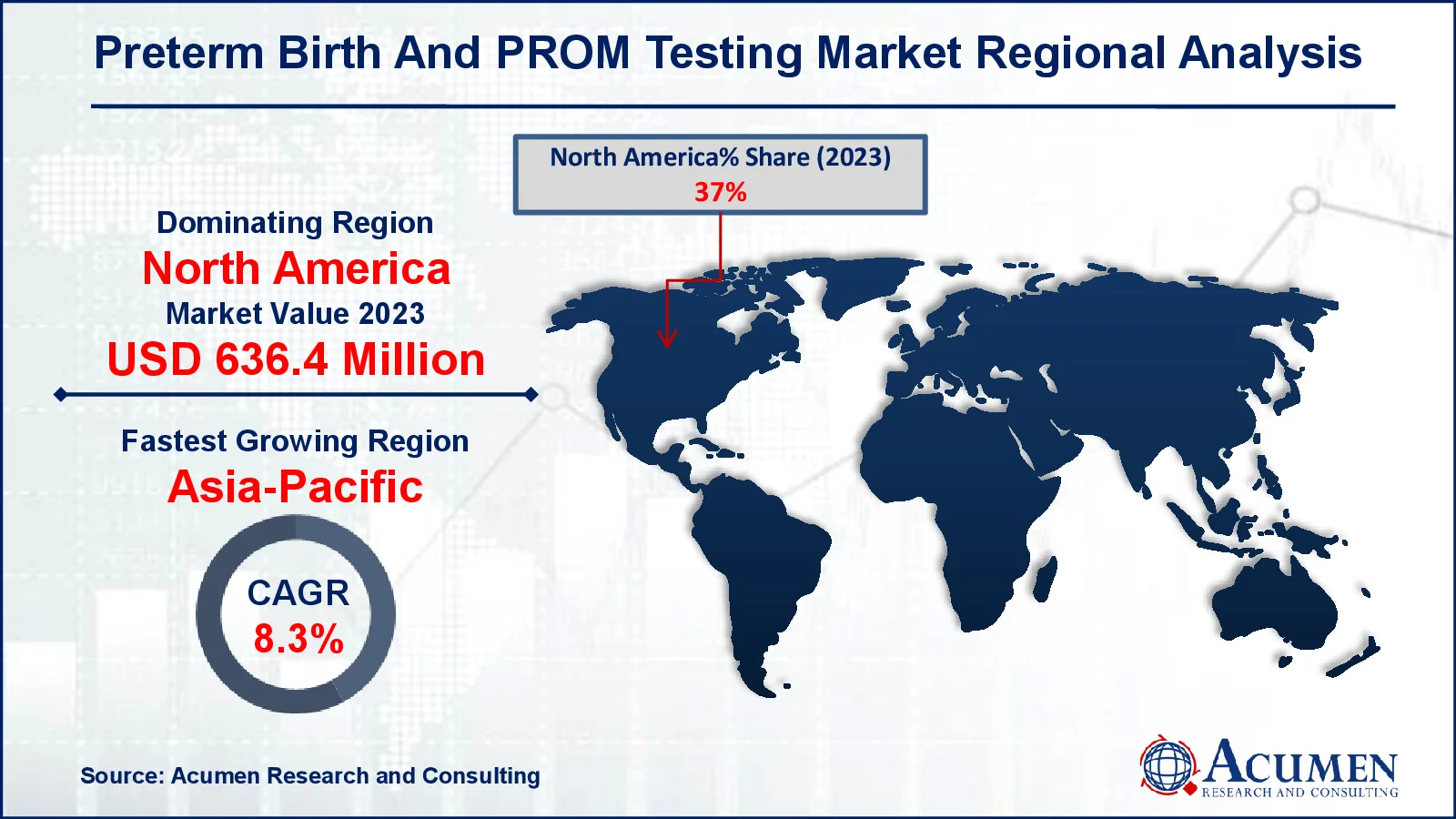
- In 2023, the North American preterm birth and PROM testing market was valued at approximately USD 636.4 billion
- The Asia-Pacific preterm birth and PROM testing market is expected to achieve a CAGR exceeding 8.3% from 2024 to 2032
- The hospitals sub-segment saw a 46% growth rate in 2023 based on end-user data
- Expanding awareness and government initiatives supporting maternal health diagnostics is the preterm birth and PROM testing market trend that fuels the industry demand
Premature rupture of membranes (PROM) is one of the leading causes of premature birth. Preterm birth testing refers to diagnostic procedures for predicting and detecting early labor, which occurs before 37 weeks of gestation and is a major cause of infant mortality and long-term health issues. Common testing includes biomarker screens, pelvic exams, uterine monitoring, and ultrasounds. PROM (Premature Rupture of Membranes) testing detects the rupture of the amniotic sac before labor begins, which can lead to premature birth or infections. Nitrazine and ferning tests, as well as fetal fibronectin assays, are all PROM tests. These diagnostics are critical for timely management in high-risk pregnancies, hence reducing problems. The applications cover hospitals, diagnostic labs, and home care, ensuring comprehensive prenatal care.
Global Preterm Birth And PROM Testing Market Dynamics
Market Drivers
- Rising preterm birth rates worldwide
- Increasing awareness of maternal and neonatal health
- Advances in diagnostic technologies for early detection
Market Restraints
- High cost of advanced diagnostic tests
- Limited availability of skilled healthcare professionals in low-resource settings
- Regulatory challenges and approval delays for new tests
Market Opportunities
- Expansion of point-of-care testing in remote areas
- Development of novel biomarkers for more accurate detection
- Growing demand for non-invasive and home-based testing solutions
Preterm Birth And PROM Testing Market Report Coverage
|
Market |
Preterm Birth And PROM Testing Market |
|
Preterm Birth And PROM Testing Market Size 2023 |
USD 1.7 Billion |
|
Preterm Birth And PROM Testing Market Forecast 2032 |
USD 3.3 Billion |
|
Preterm Birth And PROM Testing Market CAGR During 2024 - 2032 |
7.5% |
|
Preterm Birth And PROM Testing Market Analysis Period |
2020 - 2032 |
|
Preterm Birth And PROM Testing Market Base Year |
2023 |
|
Preterm Birth And PROM Testing Market Forecast Data |
2024 - 2032 |
|
Segments Covered |
By Type, By End-User, and By Geography |
|
Regional Scope |
North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
|
Key Companies Profiled |
IQ Products, Hologic, Abbott Laboratories, Cooper Surgical, Medixbiochemica, Sera Prognostics, Coopersurgical Inc., Qiagen, NX Prenatal; Inc, and Biosynex. |
|
Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Covid-19 Analysis, Regulation Analysis |
Preterm Birth And PROM Testing Market Insights
The global preterm birth and prom testing market is being driven by an increase in the number of preterm births as the average age of women becoming pregnant rises, as well as other health concerns such as diabetes and hypertension. Furthermore, the increasing need for point-of-care testing devices and the rising popularity of low-cost symptomatic tests are major factors driving global market expansion.
On the other hand, a lack of understanding about new breakthroughs in the diagnosis of preterm births, as well as the use of alternative diagnostic alternatives such as lab testing, transvaginal examination, ultrasound, uterine observation, and so on, may impede market growth.
Some factors projected to drive growth in the global market include an increase in the number of preterm births, an increase in the number of claim to fame facilities, a high rate of preterm births, and the ease with which preterm babies can be identified with PROM testing.
New diagnostic tools for preterm birth and PROM testing are being held back by regulatory obstacles and approval delays. Stringent clinical validation and safety standards might cause approval delays, limiting market penetration for novel technologies. This hinders the adoption of modern testing procedures, especially in nations with stricter regulatory regimes.
Traditional preterm birth testing methods include sterile speculum examination (pooling), pelvic examination, blood test, ultrasound, and fetal fibronectin (fFN) test. To investigate preterm labor, professionals check side effects such as pelvic weight, vaginal spotting or death, problems or normal withdrawals, vaginal release, and liquid spilling. A pelvic test is performed several times over a couple of hours to evaluate constrictions and identify changes in the cervix. Pelvic examination is the primary test used by doctors to quantify changes in the cervix.
Preterm Birth And PROM Testing Market Segmentation
The worldwide market for preterm birth and PROM testing is split based on type, end-user, and geography.
Preterm Birth And PROM Testing Market By Type
- Preterm Birth Tests
- Pelvic Exam
- Ultrasound
- Uterine Monitoring
- Biomarkers
- PROM Tests
- Nitrazine Test
- Ferning Test
- Pooling
- Ultrasound
- Fetal Fibronectin Test
- Biomarker Tests
- Others
Preterm birth tests shows robust growth in preterm birth and PROM testing market due to the critical necessity for early detection and treatment of preterm labor, which is a primary cause of infant death. Biomarker screens, ultrasounds, and uterine monitoring are increasingly being utilized to identify preterm birth risks and thereby avoid difficulties. As preterm birth rates climb worldwide, the demand for these tests outstrips PROM testing methods, which are more specific to membrane rupture detection.
Preterm Birth And PROM Testing Market By End-User
- Hospitals
- Diagnostic Laboratories
- Others
According to the preterm birth and PROM testing industry analysis, hospitals dominate industry because of their well-established infrastructure and access to cutting-edge diagnostic techniques. They work with high-risk pregnancies and complex cases that require early and reliable testing for preterm labor and membrane rupture. Furthermore, hospitals frequently act as primary referral centers, providing comprehensive prenatal care as well as a wide range of diagnostic services.
Preterm Birth And PROM Testing Market Regional Outlook
North America
- U.S.
- Canada
Europe
- U.K.
- Germany
- France
- Spain
- Rest of Europe
Asia-Pacific
- India
- Japan
- China
- Australia
- South Korea
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Rest of LATAM
The Middle East & Africa
- South Africa
- GCC Countries
- Rest of the Middle East & Africa (ME&A)
Preterm Birth And PROM Testing Market Regional Analysis
For several reasons, North America leads the preterm birth and PROM testing market due to its healthcare infrastructure, increased awareness of maternal and neonatal health, and the presence of important industry competitors. The region also benefits from favorable government policies and research funding, which encourage the development and use of innovative diagnostic technology. Every year in the North American market, approximately 3.9 million infants are conceived, with 12.0% of them born prematurely.
Asia-Pacific is predicted to lead the Preterm Birth and PROM testing market due to its expanding healthcare infrastructure, increased maternal health awareness, and rising preterm birth rates in densely populated countries such as India and China. The region's growing availability to diagnostic technology, combined with government initiatives to improve prenatal care, is propelling market expansion.
Germany has one of the highest preterm birth rates in Europe, accounting for 9.0% of all 670,000 births each year. Such circumstances highlight the need for improved, extremely precise, yet cost-effective ways for detecting premature membrane rupture.
Preterm Birth And PROM Testing Market Players
Some of the top preterm birth and PROM testing companies offered in our report include IQ Products, Hologic, Abbott Laboratories, Cooper Surgical, Medixbiochemica, Sera Prognostics, Coopersurgical Inc., Qiagen, NX Prenatal; Inc, and Biosynex.
Frequently Asked Questions
How big is the Preterm Birth And PROM Testing market?
The preterm birth and PROM testing market size was valued at USD 1.7 billion in 2023.
What is the CAGR of the global Preterm Birth And PROM Testing market from 2024 to 2032?
The CAGR of preterm birth and PROM testing is 7.5% during the analysis period of 2024 to 2032.
Which are the key players in the Preterm Birth And PROM Testing market?
The key players operating in the global market are including IQ Products, Hologic, Abbott Laboratories, Cooper Surgical, Medixbiochemica, Sera Prognostics, Coopersurgical Inc., Qiagen, NX Prenatal; Inc, and Biosynex
Which region dominated the global Preterm Birth And PROM Testing market share?
North America held the dominating position in preterm birth and PROM testing industry during the analysis period of 2024 to 2032.
Which region registered fastest CAGR from 2024 to 2032?
Asia-Pacific region exhibited fastest growing CAGR for market of preterm birth and PROM testing during the analysis period of 2024 to 2032.
What are the current trends and dynamics in the global Preterm Birth And PROM Testing industry?
The current trends and dynamics in the preterm birth and PROM testing industry include rising preterm birth rates worldwide, increasing awareness of maternal and neonatal health, and advances in diagnostic technologies for early detection.
Which end user held the maximum share in 2023?
The hospitals expected to hold the maximum share of the preterm birth and prom testing industry.?



