Neurothrombectomy Devices Market Size - Global Industry, Share, Analysis, Trends and Forecast 2022 - 2030
Published :
Report ID:
Pages :
Format :
Neurothrombectomy Devices Market Size - Global Industry, Share, Analysis, Trends and Forecast 2022 - 2030
Report Coverage
- Industry Dynamics
- Market Size and Forecast Data
- Segment Analysis
- Competitive Landscape
- Regional Analysis with a Niche Focus on Country-Level Data
- High Level Analysis - Porter's, PESTEL, Value Chain, etc.
- Company Profiles of Key Players
- Option to Customize the Report As Per Your Specific Need
Request Sample Report
The Global Neurothrombectomy Devices Market Size accounted for USD 730 Million in 2021 and is estimated to achieve a market size of USD 1,227 Million by 2030 growing at a CAGR of 6.3% from 2022 to 2030. The growing elderly population, combined with an increase in cases of high blood pressure, which results in the formation of clots and strokes, is a crucial factor positively affecting the market neurothrombectomy devices growth. Furthermore, increasing acceptance of unhealthy lifestyle habits and rising population awareness of the condition are supporting the neurothrombectomy devices market revenue.
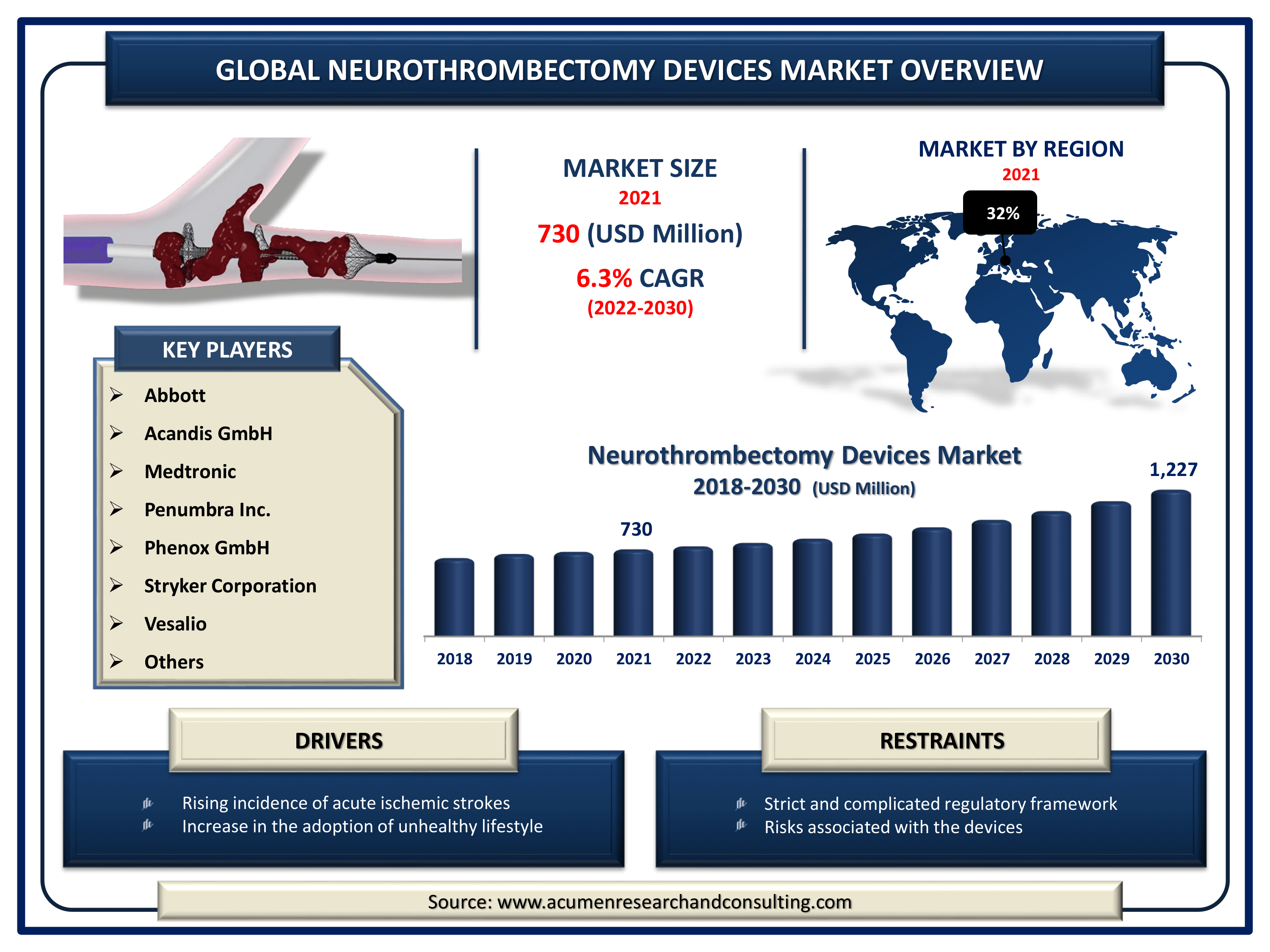
Neurothrombectomy Devices Market Report Key Highlights
- Global neurothrombectomy devices market value is estimated to expand by USD 1,227 Million by 2030, with a 6.3% CAGR from 2022 to 2030.
- Europe neurothrombectomy devices industry will record over 32% regional shares in 2021
- Asia-Pacific regional market would grow with a substantial CAGR from 2022 to 2030
- According to CDC estimates, around 795,000 individuals in the United States have a stroke each year.
- According to a recent survey, approximately 87% of all strokes are ischemic strokes, in which blood supply to the brain is obstructed
- Based on product segment, clot retriever sector will account for more than 58% of overall market share in 2021
Neurothrombectomy devices are U.S. Food and Drug Administration (FDA) approved medical devices intended to retrieve or destroy blood clots in the cerebral neurovasculature by mechanical, laser, ultrasound technologies, or a combination of technologies. Two neuro thrombectomy devices including the MERCI clot retriever and the penumbra system are FDA cleared through the FDA 510(k) process. These devices offer a number of advantages when compared to pharmacologic thrombolysis such as enhanced efficacy in treating large vessel occlusions, rapid achievement of recanalization vs IV rtPA, and greater efficacy with a lower risk for hemorrhagic events. Whereas, neuro thrombectomy devices employ a variety of mechanisms including clot retrievers, aspiration/suction devices, snare-like devices, ultrasonography technologies, and lasers.
Global Neurothrombectomy Devices Market Dynamics
Market Drivers
- Rising incidence of acute ischemic strokes
- Increase in the adoption of unhealthy lifestyle
- Growing demands for minimally invasive procedures
- Increased focus on strategic acquisitions by key players
Market Restraints
- Strict and complicated regulatory framework
- Risks associated with the devices
Market Opportunities
- Introduction of novel technologically advanced products
- Rising awareness among the population
Neurothrombectomy Devices Market Report Coverage
| Market | Neurothrombectomy Devices Market |
| Neurothrombectomy Devices Market Size 2021 | USD 730 Million |
| Neurothrombectomy Devices Market Forecast 2030 | USD 1,227 Million |
| Neurothrombectomy Devices Market CAGR During 2022 - 2030 | 6.3% |
| Neurothrombectomy Devices Market Analysis Period |
2018 - 2030 |
| Neurothrombectomy Devices Market Base Year | 2021 |
| Neurothrombectomy Devices Market Forecast Data | 2022 - 2030 |
| Segments Covered | By Product, By End-Use, And By Geography |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
| Key Companies Profiled | Abbott, Acandis GmbH, Boston Scientific Corporation, Edwards Lifesciences Corporation, Medtronic, Penumbra Inc., Phenox GmbH, Stryker Corporation, and Vesalio. |
| Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Regulation Analysis |
The increasing prevalence of acute ischemic strokes is boosting the demand for neurothrombectomy devices in the market. Acute ischemic strokes or AIS occurs when blood flow through a brain artery is blocked by a clot or a mass of thickened blood. Moreover, AIS is responsible for almost 90% of all strokes. The rising adoption of the unhealthy lifestyle that includes prolonged sitting, smoking, and obesity are supporting neurothrombectomy devices market trend. The technological advancements in the treatment procedures with the help of neurothrombectomy devices are additionally bolstering the market value. The growing awareness among the population about the treatments and increasing disposable income which is supporting the spending power of people is bolstering the market value
On the other hand, the risks associated with the neuro thrombectomy devices like failure to deploy the device or remove the clot, device breakage/fracture, perforation, dissection, and thrombus formation are likely to limit the growth to an extent over the forecast period from 2022 to 2030.
Neurothrombectomy Devices Market Segmentation
The worldwide neurothrombectomy devices market segmentation is based on the product, end-use, and geography.
Neurothrombectomy Devices Market By Product
- Clot Retrievers
- Aspiration/Suction Devices
- Vascular Snares
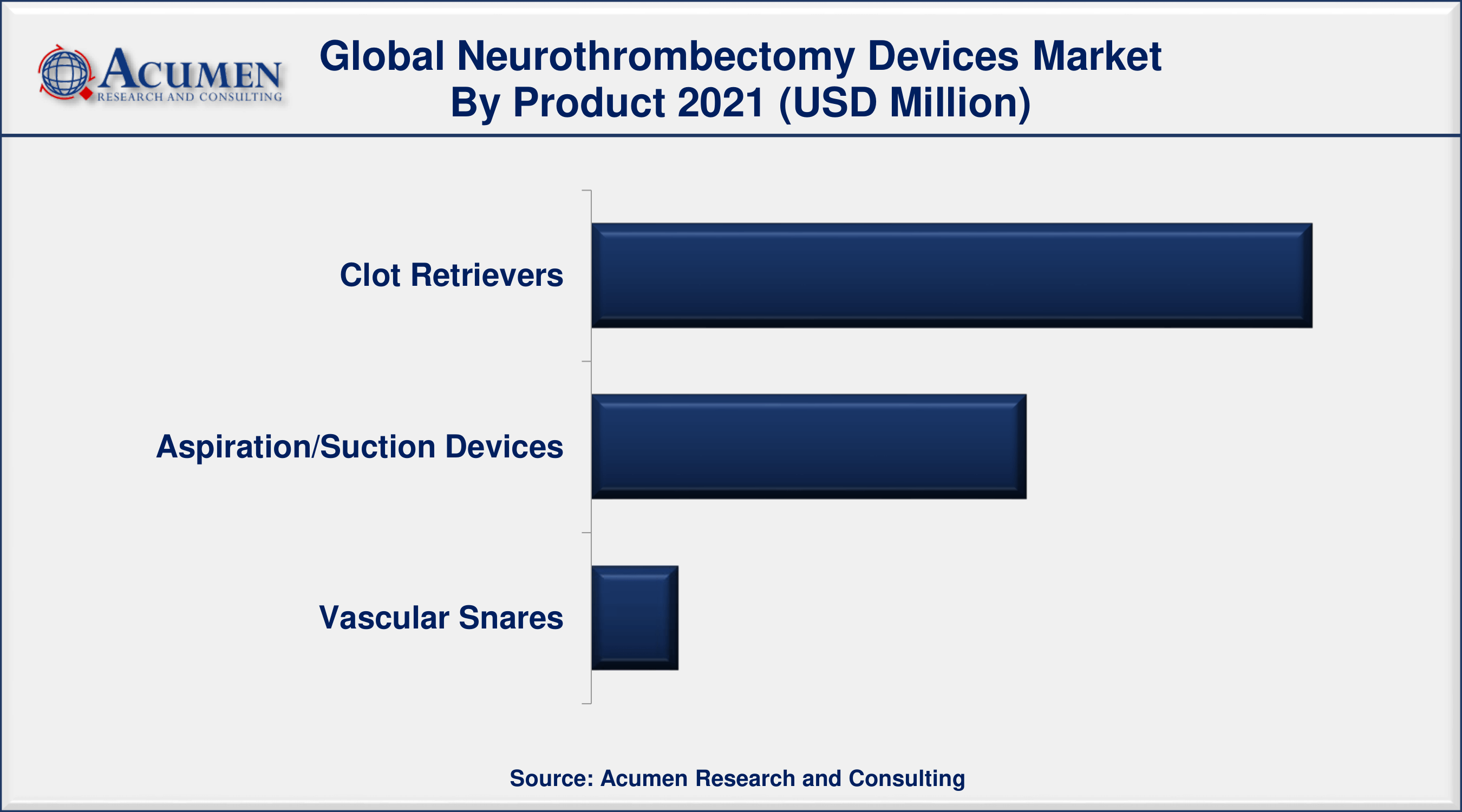
According to the neurothrombectomy devices industry analysis, the clot retriever segment dominated the market in 2021. The rising incidence of acute ischemic stroke as well as the growing number of new product releases by leading market participants are driving considerable growth in clot retrieval devices. It is primarily used to remove blood clots from cerebral arteries. Moreover, the utilization of clot retrievers has risen in geriatric persons due to the increasing occurrence of stroke. Hence, increased occurrences of the targeted condition are boosting segment growth.
Neurothrombectomy Devices Market By End-use
- Hospitals
- Emergency Clinics
- Laboratories
- Ambulatory Surgical Centers
- Research Institute
According to the neurothrombectomy devices market forecast, the hospital segment is leading the market with a major revenue share and the segment is also projected to maintain its dominance over the projected period from 2022 to 2030. As neuro thrombectomy devices require professional experience and advanced machinery for the desired task to be done successfully is the factor supporting the high usage of these devices in hospitals, where a large number of professionals exist along with the needed technical support.
Neurothrombectomy Devices Market Regional Outlook
North America
- U.S.
- Canada
Europe
- U.K.
- Germany
- France
- Spain
- Rest of Europe
Latin America
- Mexico
- Brazil
- Rest of Latin America
Asia-Pacific
- India
- Japan
- China
- Australia
- South Korea
- Rest of Asia-Pacific
The Middle East & Africa (MEA)
- Gulf Cooperation Council (GCC)
- South Africa
- Rest of the Middle East & Africa
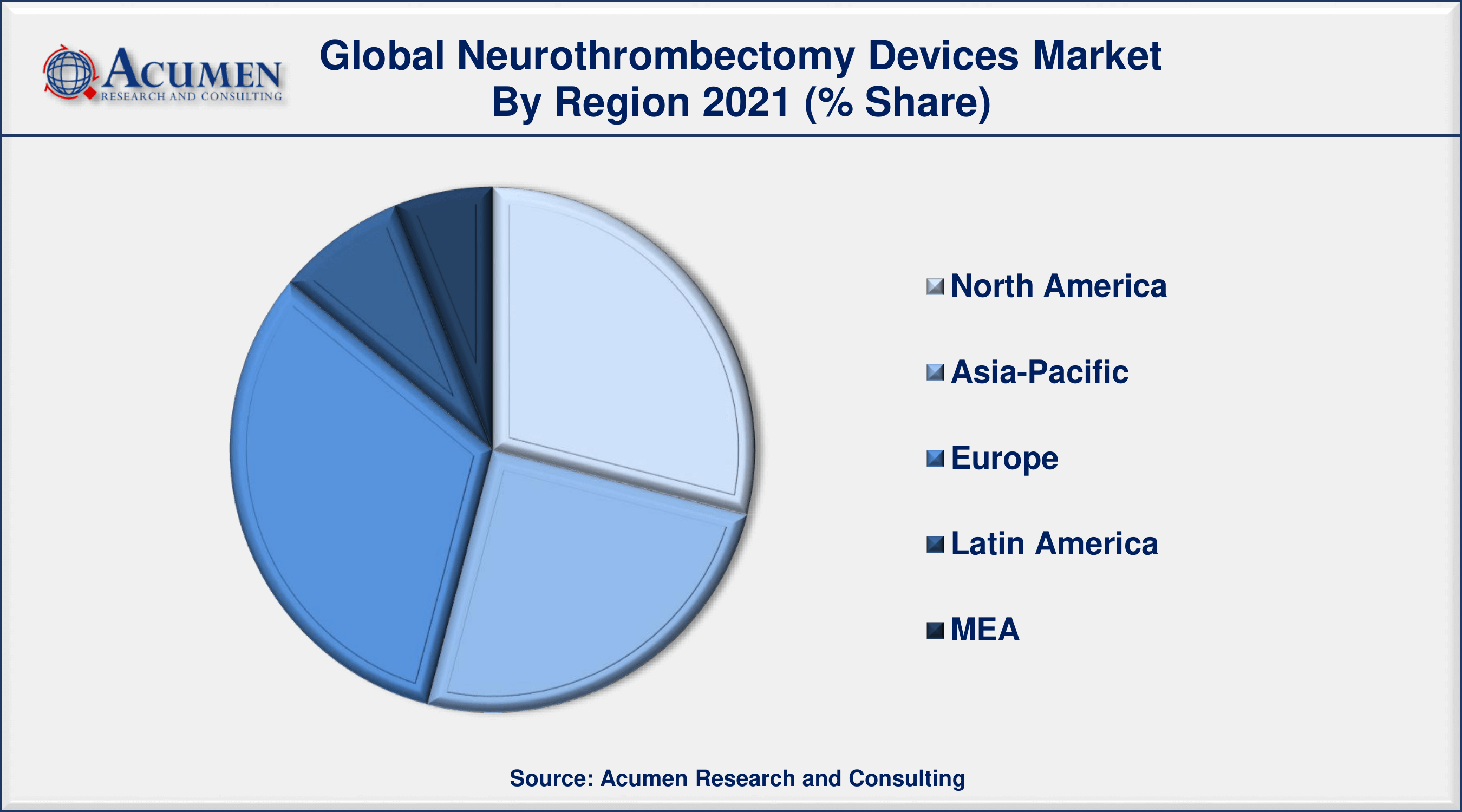
Europe Is Projected To Lead The Market With Maximum Revenue Share In The Near Future
Europe is anticipated to dominate the neurothrombectomy devices market in 2030 with a major revenue share. The presence of major players including Phenox GmbH and Acandis GmbH in the region is further estimated to support the regional market value. The continuous efforts by the major players in order to expand their market share are additionally accelerating regional market growth. The major economy of the region, Germany is expected to contribute its significant share in the future period.
Asia-Pacific Is Estimated To Experience Noticeable Growth Over The Forecast Period From 2022 To 2030
The Asia-Pacific is projected to exhibit growth over the forecast timeframe from 2022 to 2030 due to its emerging economies including China and India. Their increasing investment volume in the healthcare sector is supporting regional market growth. The presence of potential opportunities in the region due to increasing disposable income and changing lifestyles of people are bolstering the risk of stroke or blood clots in the regional population. The growing geriatric population which is prone to develop blood clots is additionally proliferating the regional market value.
Neurothrombectomy Devices Market Players
Some of the top neurothrombectomy devices market companies offered in the professional report include Abbott, Acandis GmbH, Boston Scientific Corporation, Edwards Lifesciences Corporation, Medtronic, Penumbra Inc., Phenox GmbH, Stryker Corporation, and Vesalio. 
Frequently Asked Questions
What is the size of global neurothrombectomy devices market in 2021?
The estimated value of global neurothrombectomy devices market in 2021 was accounted to be USD 730 Million.
What is the CAGR of global neurothrombectomy devices market during forecast period of 2022 to 2030?
The projected CAGR neurothrombectomy devices market during the analysis period of 2022 to 2030 is 6.3%.
Which are the key players operating in the market?
The prominent players of the global neurothrombectomy devices market are Abbott, Acandis GmbH, Boston Scientific Corporation, Edwards Lifesciences Corporation, Medtronic, Penumbra Inc., Phenox GmbH, Stryker Corporation, and Vesalio.
Which region held the dominating position in the global neurothrombectomy devices market?
Europe held the dominating neurothrombectomy devices during the analysis period of 2022 to 2030.
Which region registered the fastest growing CAGR for the forecast period of 2022 to 2030?
Asia-Pacific region exhibited fastest growing CAGR for neurothrombectomy devices during the analysis period of 2022 to 2030.
What are the current trends and dynamics in the global neurothrombectomy devices market?
Rising incidence of acute ischemic strokes, increase in the adoption of unhealthy lifestyle, and growing demands for minimally invasive procedures, drives the growth of global neurothrombectomy devices market.
By product segment, which sub-segment held the maximum share?
Based on product, clot retrievers segment is expected to hold the maximum share neurothrombectomy devices market.



