Medical Devices Vigilance Market | Acumen Research and Consulting
Medical Devices Vigilance Market Size - Global Industry, Share, Analysis, Trends and Forecast 2024 - 2032
Published :
Report ID:
Pages :
Format : 
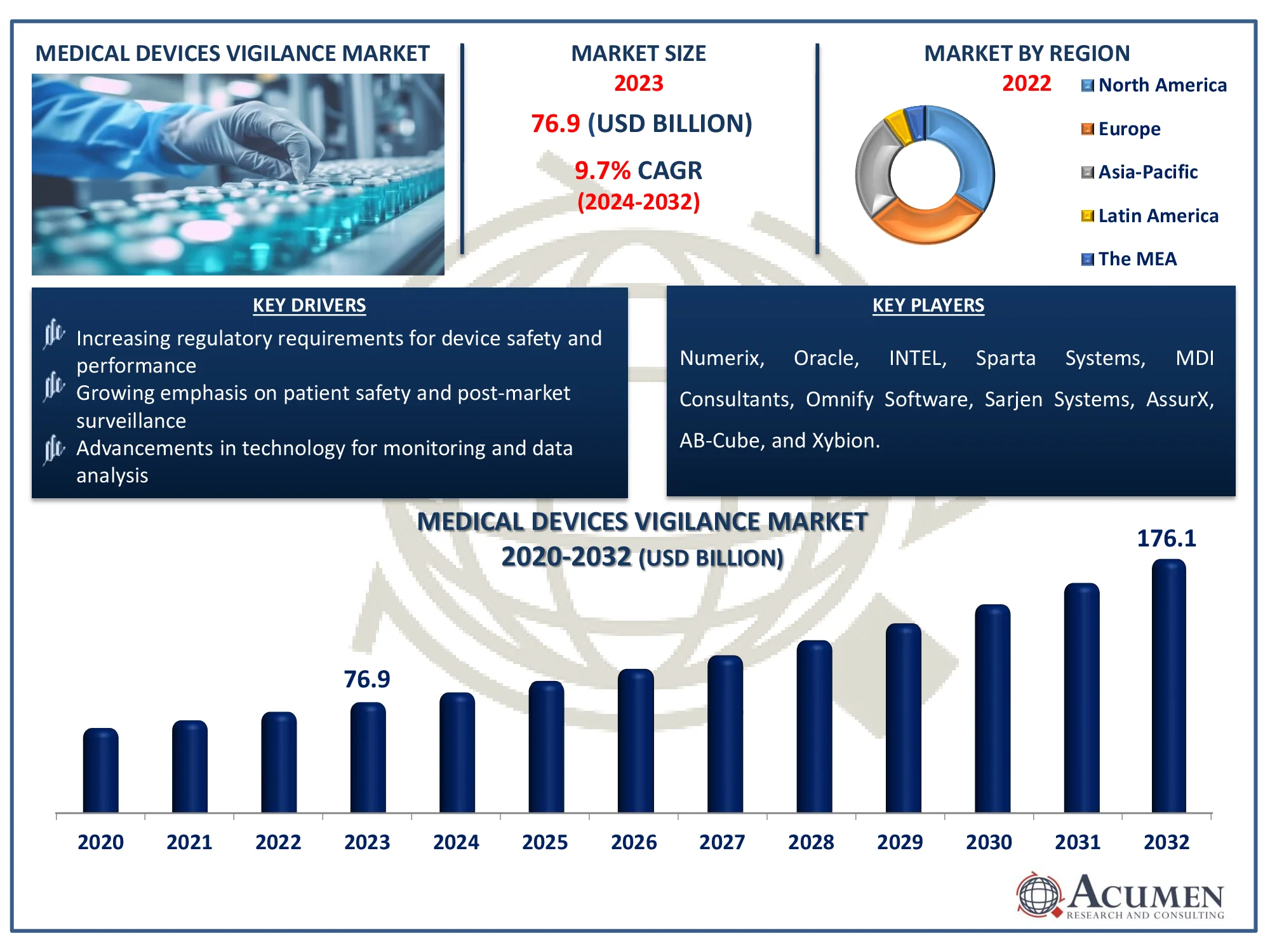
The Global Medical Devices Vigilance Market Size accounted for USD 76.9 Billion in 2023 and is estimated to achieve a market size of USD 176.1 Billion by 2032 growing at a CAGR of 9.7% from 2024 to 2032.
Medical Devices Vigilance Market (By Delivery Mode: On-Demand, On-Premises; By Application: Therapeutic, Diagnostic, Surgical, Research, and Others; By End-user: Business Process Outsourcing [BPO], Clinical Research Organizations [CROs], Original Equipment Manufacturers [OEMs], and Others and By Region: North America, Europe, Asia-Pacific, Latin America, and MEA)
Medical Devices Vigilance Market Highlights
- The global medical devices vigilance market is projected to reach USD 176.1 billion by 2032, with a CAGR of 9.7% from 2024 to 2032
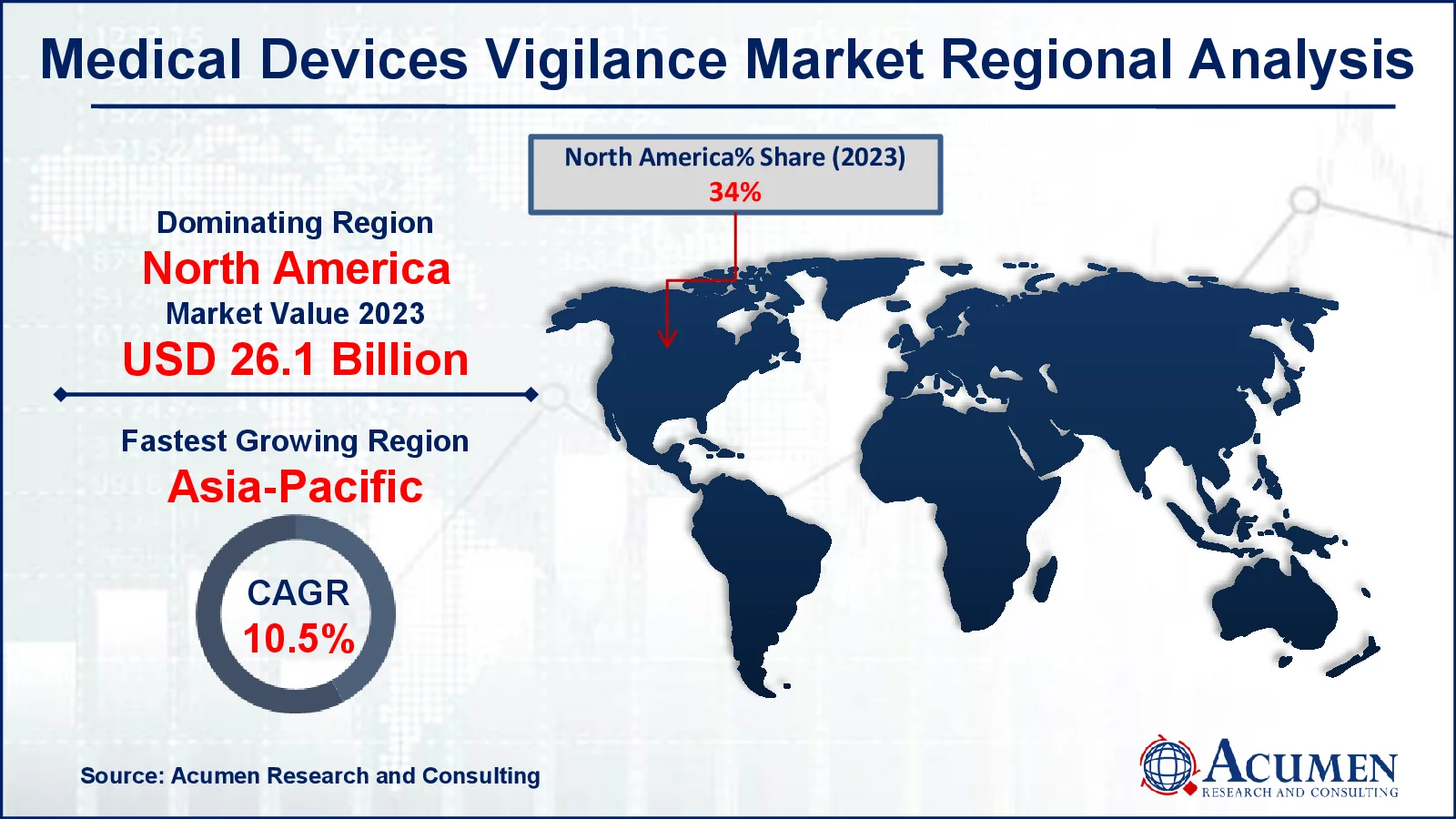
- In 2023, the North American medical devices vigilance market was valued at approximately USD 26.1 billion
- The Asia-Pacific region is expected to grow at a CAGR of over 10.5% from 2024 to 2032
- The on-demand delivery mode accounted for 81% of the market share in 2023
- The diagnostic application sub-segment captured 36% of the market share in 2023
- Clinical research organizations (CROs) represented 42% of the market share in 2023
- The rise of digital health tools is enhancing post-market surveillance capabilities is the medical devices vigilance market trend that fuels the industry demand
Medical device vigilance is the systematic process of monitoring and maintaining the safety, performance, and compliance of medical devices during their entire lifecycle. It includes monitoring adverse events, conducting post-market surveillance, and adopting corrective steps as needed. Medical device vigilance applications include adverse event reporting, which aids in the identification and resolution of safety issues; post-market surveillance, which involves continuous monitoring of device performance; and recall management, which ensures that defective devices are effectively removed from the market. Vigilance also includes field safety corrective steps to address issues discovered during actual device use. This vigilance is critical for maintaining high standards of patient safety.
Global Medical Devices Vigilance Market Dynamics
Market Drivers
- Increasing regulatory requirements for device safety and performance
- Growing emphasis on patient safety and post-market surveillance
- Advancements in technology for monitoring and data analysis
Market Restraints
- High costs associated with vigilance systems and compliance
- Complex and varied regulatory requirements across regions
- Challenges in integrating vigilance systems with existing infrastructure
Market Opportunities
- Expansion into emerging markets with growing healthcare infrastructure
- Development of AI and machine learning technologies for improved vigilance
- Increasing demand for real-time monitoring and remote surveillance solutions
Medical Devices Vigilance Market Report Coverage
| Market | Medical Devices Vigilance Market |
| Medical Devices Vigilance Market Size 2022 |
USD 76.9 Billion |
| Medical Devices Vigilance Market Forecast 2032 | USD 176.1 Billion |
| Medical Devices Vigilance Market CAGR During 2023 - 2032 | 9.7% |
| Medical Devices Vigilance Market Analysis Period | 2020 - 2032 |
| Medical Devices Vigilance Market Base Year |
2022 |
| Medical Devices Vigilance Market Forecast Data | 2023 - 2032 |
| Segments Covered | By Delivery Mode, By Application, By End-User, And By Geography |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
| Key Companies Profiled | Numerix, Oracle, INTEL, Sparta Systems, MDI Consultants, Omnify Software, Sarjen Systems, AssurX, AB-Cube, and Xybion. |
| Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Covid-19 Analysis, Regulation Analysis |
Medical Devices Vigilance Market Insights
The demand for medical device vigilance is expected to rise in the next years due to an increase in the number of adverse events reported in the United States related with medical devices. Furthermore, the use of medical device vigilance systems to provide safety to patients and healthcare workers is predicted to have a beneficial impact on the growth of the medical device vigilance industry. Furthermore, severe government medical device requirements for manufacturers to produce secure and highly effective medical equipment would support the growth of the medical device vigilance market.
The availability of vigilance software and increased patient awareness are projected to drive market expansion. Patient access to adverse event reporting allows for the transfer of files, the publication of regulatory reports, and the administration of daily medical device vigilance demands, which are all key elements that might fuel the growth of the medical device vigilance market. However, the inability of some manufacturers to assure product safety may stymie the medical device vigilance business growth over the forecast period.
Expansion into emerging economies is a substantial potential for the medical device vigilance market due to rapid expansion of healthcare infrastructure and increased acceptance of modern medical technology. For instance, according to Invest India, the Indian Medtech Industry is predicted to reach $50 billion by 2025, from an estimated $10.63 billion in 2020. The healthcare industry in India includes hospitals, medical devices, clinical trials, outsourcing, telemedicine, medical tourism, health insurance, and medical equipment. The healthcare sector is expanding rapidly due to improved coverage, services, and increased spending by both public and private entities. As these regions improve their healthcare systems, there is a greater need for comprehensive vigilance protocols to assure the safety and effectiveness of new medical equipment.
Medical Devices Vigilance Market Segmentation
The worldwide market for medical devices vigilance is split based on delivery mode, application, end-user, and geography.
Medical Device Vigilance Market By Delivery Mode
- On-Demand
- On-Premises
According to the medical devices vigilance industry analysis, the on-demand delivery option dominates industry because it allows for fast and flexible access to critical monitoring and reporting services. This mode enables healthcare professionals and manufacturers to respond to safety concerns, without delay. On-demand services are especially useful for handling real-time data and performing timely corrective actions to ensure continuous compliance and patient safety. This model also aids in optimizing resource allocation and reducing downtime, increasing its commercial appeal.
Medical Device Vigilance Market By Application
- Therapeutic
- Diagnostic
- Surgical
- Research
- Others
The diagnostic sub-segment is likely to lead the worldwide medical device vigilance market as sophisticated diagnostic technologies play an increasingly important role in patient care. As healthcare systems prioritize accurate and rapid diagnoses, the need for effective vigilance mechanisms in this area grows. Furthermore, the integration of digital health solutions and real-time monitoring improves diagnostic capabilities, which drives market expansion.
Medical Device Vigilance Market By End-user
- Business Process Outsourcing [BPO]
- Clinical Research Organizations [CROs]
- Original Equipment Manufacturers [OEMs]
- Others
According to the medical devices vigilance market forecast, clinical research organizations (CROs) are likely to dominate the industry. CROs provide specialized services like as device safety monitoring, adverse event reporting, and regulatory compliance, making them essential components of vigilance procedures. Their ability to provide comprehensive, scalable, and regulatory-compliant solutions is consistent with the growing demand for stringent device monitoring. CROs can also use their worldwide network to manage vigilance across many marketplaces efficiently.
Medical Devices Vigilance Market Regional Outlook
North America
- U.S.
- Canada
Europe
- U.K.
- Germany
- France
- Spain
- Rest of Europe
Asia-Pacific
- India
- Japan
- China
- Australia
- South Korea
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Rest of Latin America
The Middle East & Africa
- South Africa
- GCC Countries
- Rest of the Middle East & Africa (ME&A)
Medical Devices Vigilance Market Regional Analysis
For several reasons, the North American region is likely to lead the worldwide medical device vigilance market. The domination of the medical device vigilance market in North America is attributed to an increase in the number of adverse events in the United States. The medical device vigilance market is primarily driven by the demand for technological advancements and equipment, as well as favorable regulatory regulations aimed at strengthening medical device vigilance.
The Asia-Pacific region is expected to see the most growth in the medical device vigilance market, owing to developing healthcare infrastructure, increased patient safety awareness, and increased regulatory oversight. The region's expanding medical device industry, as well as government attempts to improve post-market surveillance, are driving this growth. For instance, 25,000 crores have been granted for 2023 (January to December). AB PM-JAY has empanelled 26,901 hospitals, including 11,813 private hospitals, to provide healthcare services to scheme beneficiaries. AB PM-JAY has ensured gender equality in access to healthcare services. Key markets, including China, India, and Japan, are projected to increase demand for vigilance solutions.
Medical Devices Vigilance Market Players
Some of the top medical devices vigilance companies offered in our report include Numerix, Oracle, INTEL, Sparta Systems, MDI Consultants, Omnify Software, Sarjen Systems, AssurX, AB-Cube, and Xybion.
Frequently Asked Questions
How big is the medical devices vigilance market?
The medical devices vigilance market size was valued at USD 76.9 billion in 2023.
What is the CAGR of the global medical devices vigilance market from 2024 to 2032?
The CAGR of medical devices vigilance is 9.7% during the analysis period of 2024 to 2032.
Which are the key players in the medical devices vigilance market?
The key players operating in the global market are including Numerix, Oracle, INTEL, Sparta Systems, MDI Consultants, Omnify Software, Sarjen Systems, AssurX, AB-Cube, and Xybion.
Which region dominated the global medical devices vigilance market share?
North America held the dominating position in medical devices vigilance industry during the analysis period of 2024 to 2032.
Which region registered fastest CAGR from 2024 to 2032?
Asia-Pacific region exhibited fastest growing CAGR for market of medical devices vigilance during the analysis period of 2024 to 2032.
What are the current trends and dynamics in the global medical devices vigilance industry?
The current trends and dynamics in the medical devices vigilance industry include increasing regulatory requirements for device safety and performance, growing emphasis on patient safety and post-market surveillance, and advancements in technology for monitoring and data analysis.
Which delivery mode held the maximum share in 2023?
The on-demand delivery mode held the maximum share of the medical devices vigilance industry.
Select Licence Type
Connect with our sales team
Why Acumen Research And Consulting
100%
Customer Satisfaction
24x7
Availability - we are always there when you need us
200+
Fortune 50 Companies trust Acumen Research and Consulting
80%
of our reports are exclusive and first in the industry
100%
more data and analysis
1000+
reports published till date



