Clinical Trial Supply and Logistics Market Size - Global Industry, Share, Analysis, Trends and Forecast 2023 - 2032
Published :
Report ID:
Pages :
Format :
Clinical Trial Supply and Logistics Market Size - Global Industry, Share, Analysis, Trends and Forecast 2023 - 2032
Report Coverage
- Industry Dynamics
- Market Size and Forecast Data
- Segment Analysis
- Competitive Landscape
- Regional Analysis with a Niche Focus on Country-Level Data
- High Level Analysis - Porter's, PESTEL, Value Chain, etc.
- Company Profiles of Key Players
- Option to Customize the Report As Per Your Specific Need
Request Sample Report
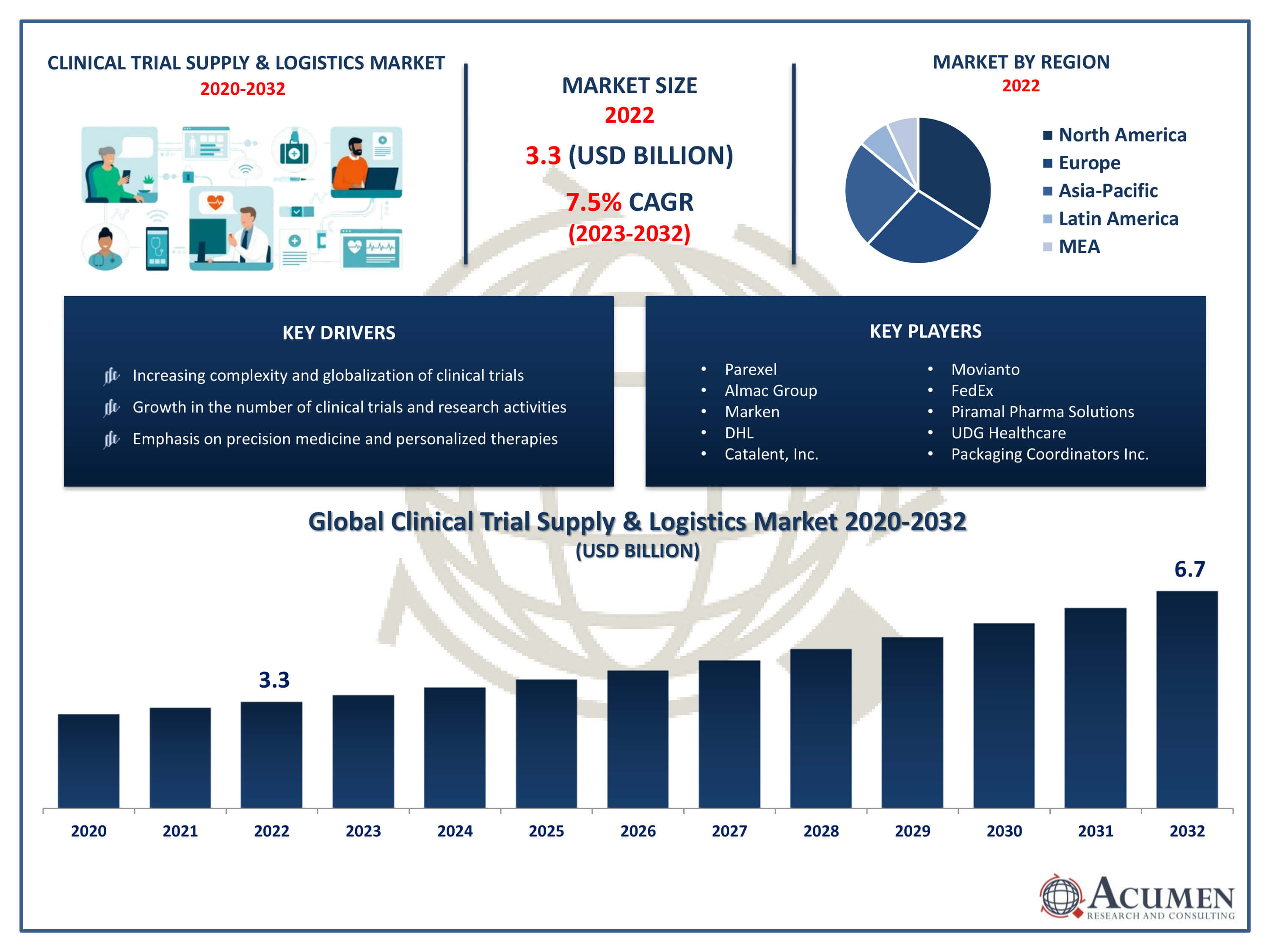
The Global Clinical Trial Supply and Logistics Market Size accounted for USD 3.3 Billion in 2022 and is projected to achieve a market size of USD 6.7 Billion by 2032 growing at a CAGR of 7.5% from 2023 to 2032.
Clinical Trial Supply and Logistics Market Highlights
- Global Clinical Trial Supply and Logistics Market revenue is expected to increase by USD 6.7 Billion by 2032, with a 7.5% CAGR from 2023 to 2032
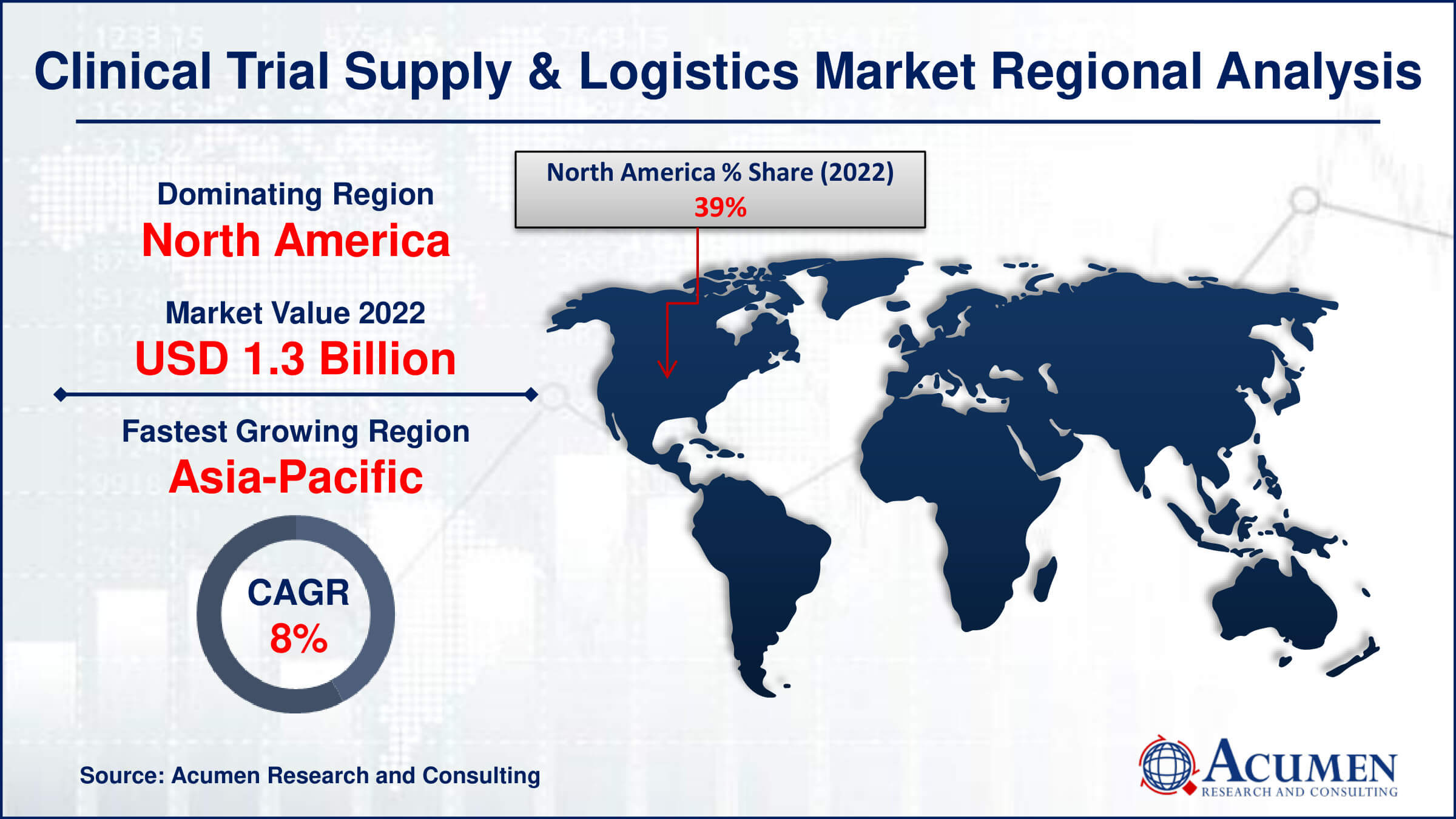
- North America region led with more than 39% of Clinical Trial Supply and Logistics Market share in 2022
- Asia-Pacific Clinical Trial Supply and Logistics Market growth will record a CAGR of around 8.1% from 2023 to 2032
- By service, the logistics & distribution segment is the largest segment in the market, accounting for over 25% of the market share in 2022
- By end-user, the pharmaceuticals segment has recorded more than 44% of the revenue share in 2022
- Increasing complexity and globalization of clinical trials, drives the Clinical Trial Supply and Logistics Market value
Clinical trial supply and logistics refer to the planning, management, and distribution of investigational drugs, medical devices, and other materials required for clinical trials. The process involves ensuring that the right quantity of the investigational product reaches the right location at the right time while maintaining compliance with regulatory requirements. This includes managing the entire supply chain from manufacturing to distribution and addressing challenges such as temperature control, regulatory compliance, and global distribution.
The market for clinical trial supply and logistics has been experiencing growth due to several factors. The increasing number of clinical trials globally, driven by advancements in medical research and the demand for innovative therapies, has led to a rise in the need for efficient supply chain management. Additionally, the complexity of clinical trials has increased with the globalization of research, requiring specialized expertise in managing the logistics of shipping and storing investigational products. The COVID-19 pandemic has also highlighted the importance of resilient and adaptable supply chains in the pharmaceutical and biotechnology industries, further emphasizing the significance of robust clinical trial supply and logistics services.
Global Clinical Trial Supply and Logistics Market Trends
Market Drivers
- Increasing complexity and globalization of clinical trials
- Growth in the number of clinical trials and research activities
- Emphasis on precision medicine and personalized therapies
- Rising demand for specialized temperature-controlled logistics
- Impact of the COVID-19 pandemic on supply chain resilience
Market Restraints
- Stringent regulatory requirements and compliance challenges
- High costs associated with temperature-sensitive logistics
Market Opportunities
- Adoption of advanced technologies, such as blockchain and IoT, for supply chain optimization
- Increasing focus on patient-centric clinical trial designs
Clinical Trial Supply & Logistics Market Report Coverage
| Market | Clinical Trial Supply and Logistics Market |
| Clinical Trial Supply and Logistics Market Size 2022 | USD 3.3 Billion |
| Clinical Trial Supply and Logistics Market Forecast 2032 | USD 6.7 Billion |
| Clinical Trial Supply and Logistics Market CAGR During 2023 - 2032 | 7.5% |
| Clinical Trial Supply and Logistics Market Analysis Period | 2020 - 2032 |
| Clinical Trial Supply and Logistics Market Base Year |
2022 |
| Clinical Trial Supply and Logistics Market Forecast Data | 2023 - 2032 |
| Segments Covered | By Service, By Phase, By Therapeutic Area, By End-user, And By Geography |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
| Key Companies Profiled | Parexel, Almac Group, Marken, DHL, Catalent, Inc., Movianto, FedEx, Piramal Pharma Solutions, UDG Healthcare, Packaging Coordinators Inc., and Thermo Fisher Scientific. |
| Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Covid-19 Analysis, Regulation Analysis |
Clinical trial supply and logistics involve the planning, execution, and management of the entire supply chain for investigational drugs, medical devices, and other materials required during clinical trials. This process is critical to ensuring that the right quantity of the investigational product reaches the right location at the right time, meeting the stringent regulatory requirements governing the conduct of clinical trials. Clinical trial supply and logistics cover various stages, including manufacturing, packaging, labeling, storage, distribution, and return or destruction of unused materials. The applications of clinical trial supply and logistics are diverse and integral to the successful conduct of clinical trials. Efficient supply chain management enables the timely initiation and completion of clinical trials by ensuring that investigational products are available at clinical sites when needed. This is particularly crucial in multi-center trials conducted across different geographic locations.
The clinical trial supply and logistics market have been experiencing robust growth driven by several key factors. The increasing number of clinical trials globally, particularly in the fields of pharmaceuticals and biotechnology, has created a significant demand for efficient supply chain management. The growth of personalized medicine and the development of innovative therapies have further fueled this demand, requiring the careful handling and transportation of diverse investigational products. As the complexity of clinical trials continues to rise, with more stringent regulatory requirements and a focus on precision medicine, the need for specialized supply and healthcare logistics services has become paramount. The COVID-19 pandemic has also played a role in shaping the growth of the market. The heightened awareness of the importance of clinical research, coupled with the urgency to develop and distribute vaccines and treatments, has underscored the critical role of a well-functioning and adaptable clinical trial supply chain.
Clinical Trial Supply and Logistics Market Segmentation
The global Clinical Trial Supply & Logistics Market segmentation is based on service, phase, therapeutic area, end-user, and geography.
Clinical Trial Supply and Logistics Market By Service
- Logistics & Distribution
- Packaging, Labeling, and Blinding
- Storage & Retention
- Comparator Sourcing
- Manufacturing
- Others
According to the clinical trial supply & logistics industry analysis, the logistics & distribution segment accounted for the largest market share in 2022. As clinical trials become more globalized and complex, there is an increasing need for efficient and reliable logistics solutions to transport investigational products across different regions. This growth is fueled by the rising number of international clinical trials, necessitating the timely and secure movement of pharmaceuticals, biologics, and medical devices to various trial sites. The logistics and distribution segment plays a crucial role in ensuring that these materials reach their destinations in compliance with regulatory requirements and within specified timelines. The COVID-19 pandemic has further underscored the importance of a robust logistics and distribution infrastructure in the clinical trial supply chain.
Clinical Trial Supply and Logistics Market By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
In terms of phases, the phase III segment is expected to witness significant growth in the coming years. Phase III clinical trials are large-scale studies designed to assess the safety and efficacy of investigational drugs or treatments on a broader patient population. As these trials involve a larger number of participants across multiple sites globally, the demand for efficient and well-coordinated supply chain logistics becomes paramount. The growth in Phase III clinical trials can be attributed to the increasing focus on bringing promising therapies to market and obtaining regulatory approvals, prompting pharmaceutical and biotech companies to invest significantly in this final stage of clinical development. The complexity of Phase III trials, involving diverse therapeutic areas and a wide range of investigational products, necessitates specialized supply chain and logistics solutions.
Clinical Trial Supply and Logistics Market By Therapeutic Area
- Oncology
- CNS and Mental Disorders
- Respiratory Diseases
- Cardiovascular Diseases
- Others
According to the clinical trial supply & logistics market forecast, the oncology segment is expected to witness significant growth in the coming years. Oncology clinical trials have become more prevalent due to advancements in understanding the molecular basis of cancer, leading to the discovery of targeted therapies and immunotherapies. The demand for specialized logistics solutions in oncology trials arises from the need to transport and manage complex investigational products, including chemotherapy drugs, biologics, and personalized medicine, which often have specific storage and handling requirements. The growth of the oncology segment is further fueled by the expansion of clinical trials in emerging markets, where there is a rising prevalence of cancer and a growing patient pool.
Clinical Trial Supply and Logistics Market By End-user
- Pharmaceuticals
- Medical Device
- Biologicals
Based on the end-user, the pharmaceuticals segment is expected to continue its growth trajectory in the coming years. The pharmaceutical industry is characterized by a dynamic landscape with a multitude of therapeutic areas, and as companies focus on developing novel drugs and therapies, the demand for efficient and specialized supply chain solutions has increased. The pharmaceuticals segment encompasses a wide range of investigational products, including small molecules and biologics, each with distinct storage, handling, and transportation requirements, thereby necessitating tailored logistics solutions. The global nature of pharmaceutical clinical trials, with studies conducted across multiple regions and countries, has further intensified the need for effective supply chain and logistics management. The growth of the pharmaceuticals segment is also propelled by the increasing complexity of clinical trial protocols, often involving intricate study designs and diverse patient populations.
Clinical Trial Supply and Logistics Market Regional Outlook
North America
- U.S.
- Canada
Europe
- U.K.
- Germany
- France
- Spain
- Rest of Europe
Asia-Pacific
- India
- Japan
- China
- Australia
- South Korea
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Rest of Latin America
The Middle East & Africa
- South Africa
- GCC Countries
- Rest of the Middle East & Africa (ME&A)
Clinical Trial Supply & Logistics Market Regional Analysis
North America stands out as a dominating region in the clinical trial supply and logistics market due to several key factors that contribute to its leadership position. The region boasts a robust and mature pharmaceutical and biotechnology industry, with a high concentration of leading research institutions, pharmaceutical companies, and contract research organizations (CROs). The United States, in particular, serves as a hub for clinical research and innovation, hosting a significant number of clinical trials across various therapeutic areas. This concentration of research activities has led to a heightened demand for sophisticated and efficient clinical trial supply and logistics services, driving the growth of the market in North America. Furthermore, North America's dominance in the clinical trial supply and logistics market is reinforced by its well-established infrastructure, advanced technological capabilities, and a comprehensive regulatory framework that ensures compliance with industry standards. The region's logistical advantages, including well-connected transportation networks and established cold chain capabilities, make it an ideal environment for managing the complex distribution requirements of investigational products. The COVID-19 pandemic has further underscored the resilience and adaptability of the North American clinical trial supply chain, as the region played a pivotal role in the rapid deployment of vaccines and treatments.
Clinical Trial Supply and Logistics Market Player
Some of the top clinical trial supply & logistics market companies offered in the professional report include Parexel, Almac Group, Marken, DHL, Catalent, Inc., Movianto, FedEx, Piramal Pharma Solutions, UDG Healthcare, Packaging Coordinators Inc., and Thermo Fisher Scientific.
Frequently Asked Questions
How big is the clinical trial supply and logistics market?
The clinical trial supply and logistics market size was USD 3.3 Billion in 2022.
What is the CAGR of the global clinical trial supply and logistics market from 2023 to 2032?
The CAGR of clinical trial supply and logistics is 7.5% during the analysis period of 2023 to 2032.
Which are the key players in the clinical trial supply and logistics market?
The key players operating in the global market are including Parexel, Almac Group, Marken, DHL, Catalent, Inc., Movianto, FedEx, Piramal Pharma Solutions, UDG Healthcare, Packaging Coordinators Inc., and Thermo Fisher Scientific.
Which region dominated the global clinical trial supply and logistics market share?
North America held the dominating position in clinical trial supply and logistics industry during the analysis period of 2023 to 2032.
Which region registered fastest CAGR from 2023 to 2032?
Asia-Pacific region exhibited fastest growing CAGR for market of clinical trial supply and logistics during the analysis period of 2023 to 2032.
What are the current trends and dynamics in the global clinical trial supply & logistics industry?
The current trends and dynamics in the clinical trial supply and logistics industry include increasing complexity and globalization of clinical trials, growth in the number of clinical trials and research activities, and emphasis on precision medicine and personalized therapies.
Which phase held the maximum share in 2022?
The phase III held the maximum share of the clinical trial supply and logistics industry.


