Scleroderma Therapeutics Market Size - Global Industry, Share, Analysis, Trends and Forecast 2022 - 2030
Published :
Report ID:
Pages :
Format :
Scleroderma Therapeutics Market Size - Global Industry, Share, Analysis, Trends and Forecast 2022 - 2030
Report Coverage
- Industry Dynamics
- Market Size and Forecast Data
- Segment Analysis
- Competitive Landscape
- Regional Analysis with a Niche Focus on Country-Level Data
- High Level Analysis - Porter's, PESTEL, Value Chain, etc.
- Company Profiles of Key Players
- Option to Customize the Report As Per Your Specific Need
Request Sample Report
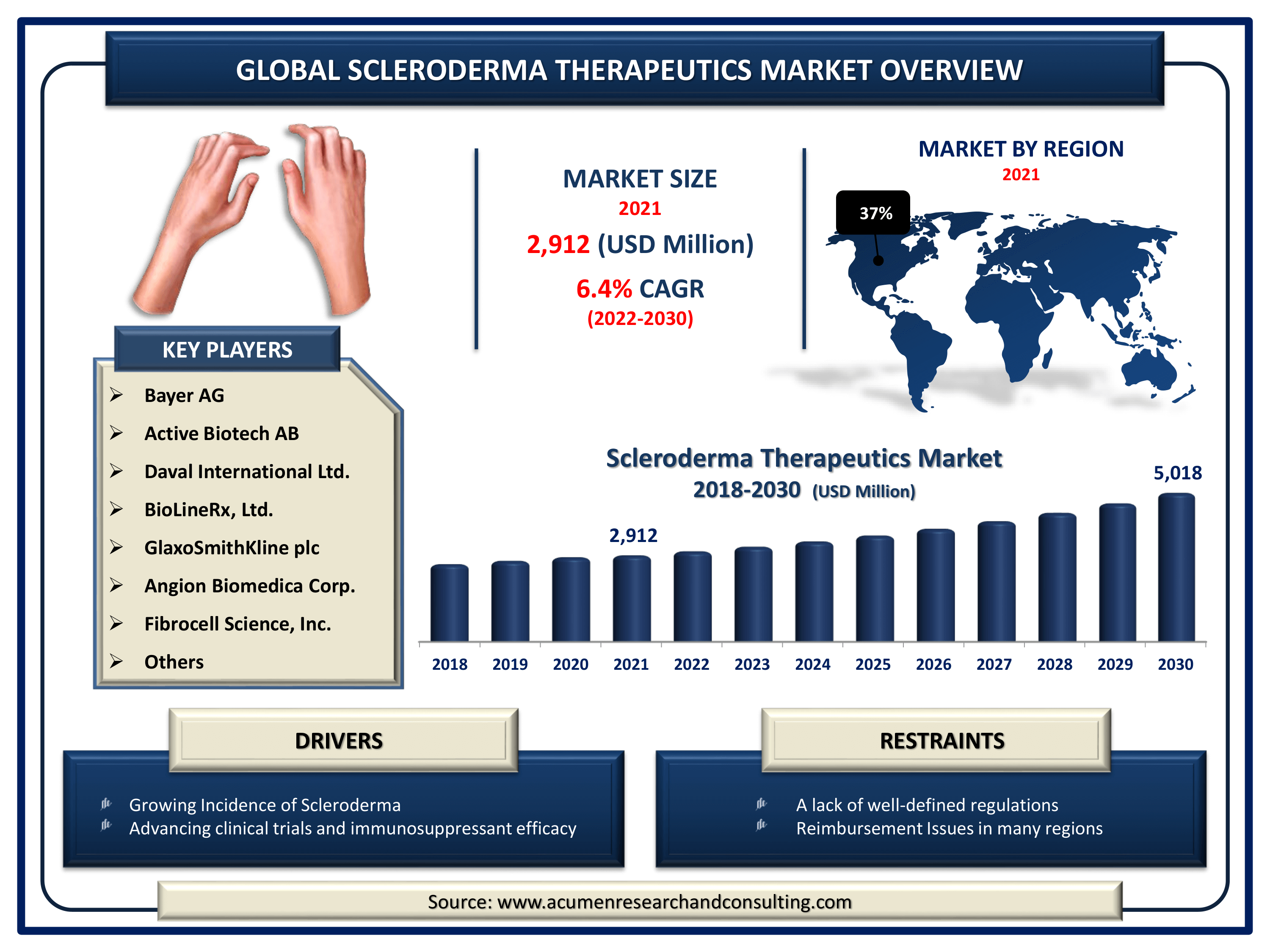
The Global Scleroderma Therapeutics Market Size accounted for USD 2,912 Million in 2021 and is estimated to achieve a market size of USD 5,018 Million by 2030 growing at a CAGR of 6.4% from 2022 to 2030. The increased incidence and screening rates of scleroderma are the key drivers of the scleroderma therapeutics market value. Moreover, the existence of a well-defined regulatory framework that supports the development of effective medicines, as well as increased frequencies of genetic abnormalities coupled with significant changes in the environment, are projected to be major elements propelling the scleroderma therapeutics market growth.
Scleroderma Therapeutics Market Report Key Highlights
- Global scleroderma therapeutics market revenue is expected to expand by USD 5,018 million by 2030, with a 6.4% CAGR from 2022 to 2030
- North America scleroderma therapeutics market share accounting for around 37% of the total market in 2021
- According to research, systemic sclerosis affects approximately 30 people per million people each year, with an estimated 125,000 active cases in the United States and maybe 2.5 million worldwide
- According to Scleroderma & Raynaud's UK, approximately 19,000 persons were diagnosed with Scleroderma in the UK in 2018
- Among drug class, immunosuppressors segment will account for more than 60% of overall market share in 2021
- By indications, systemic segment accounted 65% revenue share in 2021
Scleroderma is an autoimmune rheumatic condition that causes the skin and connective tissues to get chronically hardened and tightened. However, it includes the overproduction of collagen. The exact cause of Scleroderma is not known. Inner bodies such as blood vessels, heart, lungs, belly, and kidneys may suffer harm. Scleroderma incidents in females are four times higher than in males. In addition, starting between 30 and 50 years is more frequent.
The elevated effect on scleroderma diagnostics and on the therapy industry of divers is the increased incidence of scleroderma and the increased incidence of genetic mutations combined with dramatic environmental modifications. The incidence of systemic scleroderma is approximately 35 instances per 1 million adults according to international reports from Japan and Britain. Four times more women than males are susceptible to systemic scleroderma. Furthermore, this market is anticipated to serve as future growth possibilities if high unsatisfied medical demands and the subsequent introduction of fresh products are met.
Global Scleroderma Therapeutics Market Dynamics
Market Drivers
- Growing incidence of scleroderma
- Increasing pharmaceutical companies' focus on developing novel drugs
- Rising rates of genetic mutations, as well as extreme environmental changes
- Advancing clinical trials and immunosuppressant efficacy
Market Restraints
- A lack of well-defined regulations
- Reimbursement Issues in many regions
Market Opportunities
- Increasing research and development activities
- Expansion of the pharmaceutical pipeline
Scleroderma Therapeutics Market Report Coverage
| Market | Scleroderma Therapeutics Market |
| Scleroderma Therapeutics Market Size 2021 | USD 2,912 Million |
| Scleroderma Therapeutics Market Forecast 2030 | USD 5,018 Million |
| Scleroderma Therapeutics Market CAGR During 2022 - 2030 | 6.4% |
| Scleroderma Therapeutics Market Analysis Period | 2018 - 2030 |
| Scleroderma Therapeutics Market Base Year | 2021 |
| Scleroderma Therapeutics Market Forecast Data | 2022 - 2030 |
| Segments Covered | By Drug Class, By Indication, And By Geography |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
| Key Companies Profiled | Active Biotech AB, Daval International Ltd., BioLineRx, Ltd., Corbus pharmaceuticals, Inc., F. Hoffmann-La Roche Ltd., GlaxoSmithKline plc, Angion Biomedica Corp., Fibrocell Science, Inc., Bayer AG, Bristol-Myers Squibb Company, Allergan, Inc., and Dynavax Technologies Corporation. |
| Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Regulation Analysis |
The scleroderma therapeutics market is motivated primarily by the labeling of drugs for its symptoms such as rheumatoid arthritis that are approved. The absence of curative therapies and the elevated incidence of off-label drug use are factors that stimulate interest in the markets for rare diseases.
The development of targeted biologics and tiny molecular combination therapies is anticipated to be fuel the scleroderma therapeutics market trend. The market is also anticipated to be driven by increased scleroderma and the increasing incidence of genetic mutations combined with dramatic environmental modifications.
Furthermore, growing available revenue, allowing for increased expenditure ability and the existence of a well-defined legislative structure to support efficient therapy development in advanced region fuel market development. In addition, the development of premium curatives undergoing clinical development will increase market growth over the forecast era. Additional approvals will also enhance the development of current therapy alternatives.
This disease involves a broad variety of drug classes prescribed for first and second-line treatment. The disease is correlated with comorbidity. Among the signs, the highest possible sales were achieved with pulmonary arterial hypertension (PAH). More than one drug class and several branded drugs form part of the drugs for this comorbidity. In addition, both authorized medicines are used to target PAH in this market, namely bosentan, and iloprost. Increased Scleroderma incidence mainly drives development on the market. Scleroderma is a disease of long duration that demands a daily dose of medicines. In addition, the scleroderma diagnostics and therapeutics market is projected to raise awareness of preventive health care and boost disposable income among the population. In addition, strict public regulations, adverse drug effects, elevated healthcare costs, and Scleroderma diagnostic tests are key obstacles to the development of the industry. Ongoing R&D is expected to generate fresh possibilities during the forecast era in scleroderma diagnosis and therapeutics.
Scleroderma Therapeutics Market Segmentation
The worldwide scleroderma therapeutics market segmentation is based on the drug class, indication, and geography.
Scleroderma Therapeutics Market By Drug Class
- Immunosuppressors
- Analgesic
- Phosphodiesterase 5 Inhibitors - PHA
- Endothelin Receptor Antagonists
- Calcium Channel Blockers
- Prostacyclin Analogues
- Others
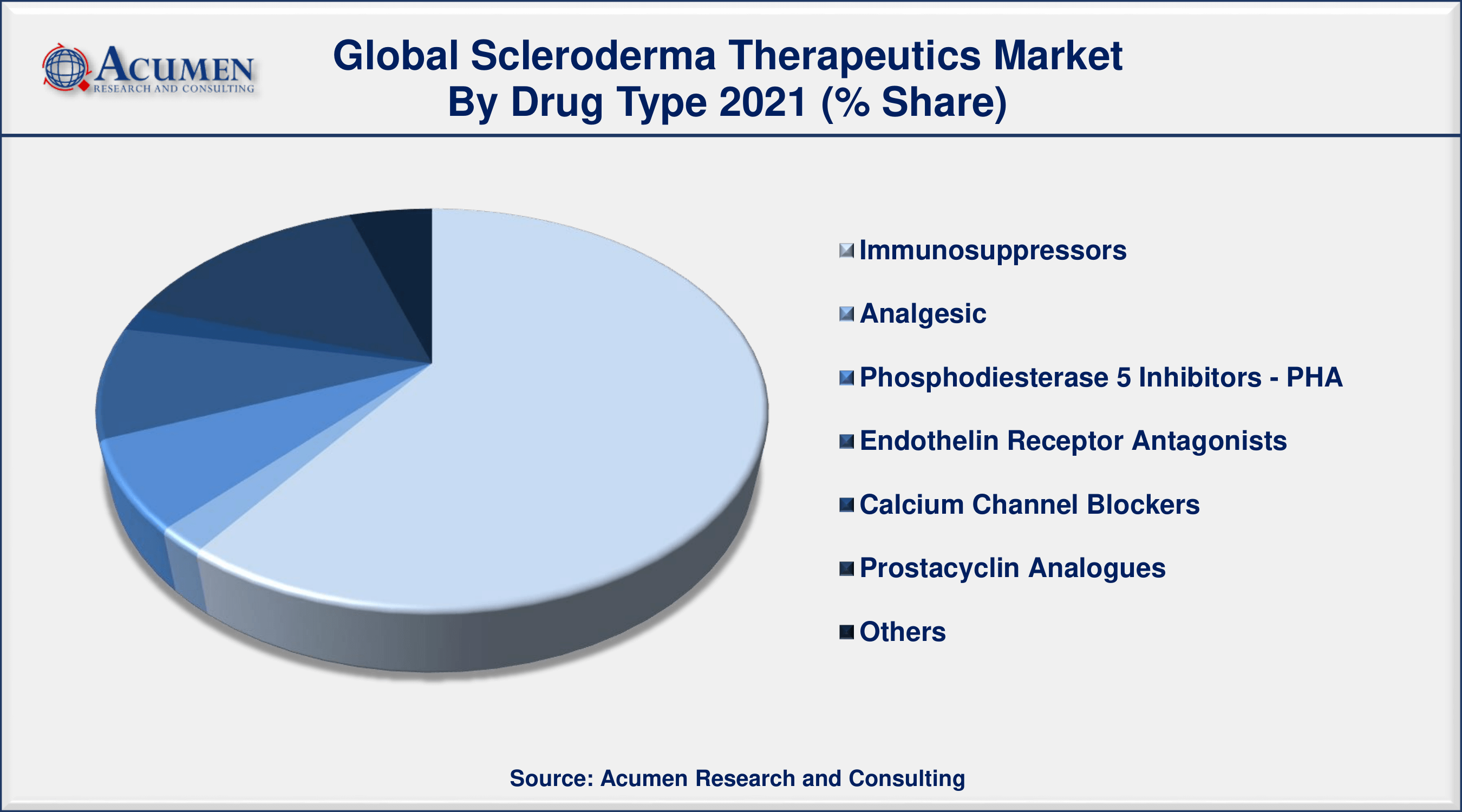
According to the scleroderma therapeutics industry analysis, the immunosuppressors segment will be the most important in the market. Immunosuppressants are of prominence because they include several biological products from well-known players like Roche. Small molecule immunosuppressants and biologics are an increasing sector with several scleroderma pipeline medicines. In the forecast period classified under "other drug classes," new treatments, including Lenabasum and Ofev, are anticipated. PDE-5 drug class market inhibitors face headwinds as a result of Revatio's patent expiry which decreases income. ERAs continue to be focused as the drug class mainly includes branded therapies and is therefore highly effective in their use in the therapy of the indication.
Scleroderma Therapeutics Market By Indication
- Systemic
- Localized
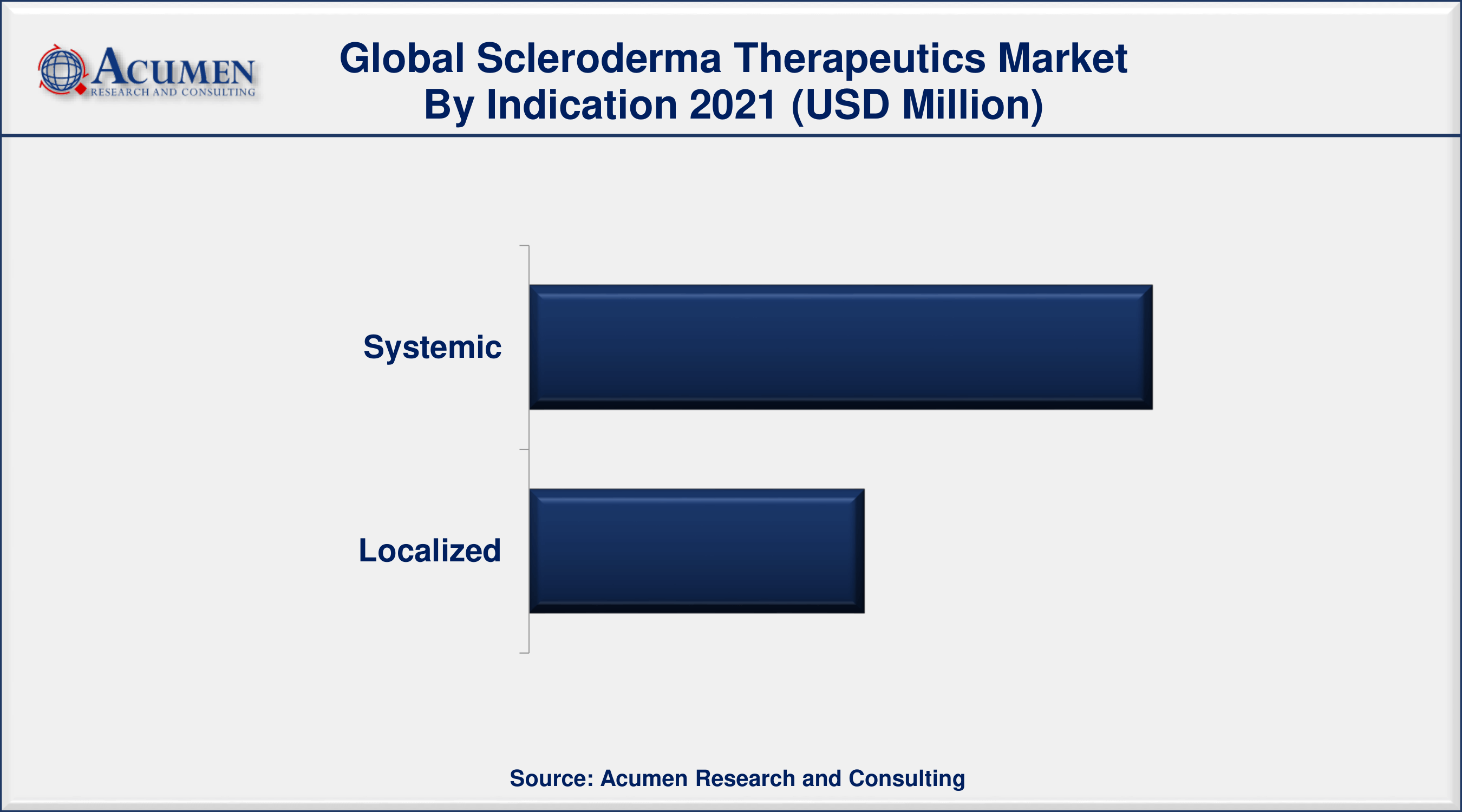
According to the scleroderma therapeutics market forecast, the systemic segment is projected to lead the market in the coming years. Systemic scleroderma symptoms and conditions range from interstitial pulmonary illness to arterial pulmonary hypertension. Most systemic symptoms are caused by the use of expensive immunosuppressants.
Local scleroderma is presently low but is anticipated to develop during the forecast period, with Fibrocell's anticipated launch of first-class FCX-013 treatment. Fibrocell's FCX-013 retains the designation of orphan drug and quick track, which would be the first localized scleroderma gene treatment if approved. This therapy is intended to stop fibrosis in the early phases. The first oral treatment of Lenabasum with the elevated curative capacity for diffuse systemic scleratic cutaneous diseases associated with an elevated co-morbidity burden is Corbus Pharmaceuticals.
Scleroderma Therapeutics Market Regional Outlook
North America
- U.S.
- Canada
Europe
- U.K.
- Germany
- France
- Spain
- Rest of Europe
Latin America
- Mexico
- Brazil
- Rest of Latin America
Asia-Pacific
- India
- Japan
- China
- Australia
- South Korea
- Rest of Asia-Pacific
The Middle East & Africa (MEA)
- Gulf Cooperation Council (GCC)
- South Africa
- Rest of the Middle East & Africa
North America Leads the Global Scleroderma Therapeutics Market
Geographically, North America dominated the global market in 2021. The main driver of income is access to newly established immunosuppressant substances and a favorable scenario of reimbursement. Europe is traveling with less of an out-of-label treatment of scleroderma than the United States mainly because of the greater use of generics and biosimilars. Generics and OTC medicines are the basis of scleroderma treatment and its symptoms in developing countries. Although prices are being cut in Japan, fresh orphan therapies can be accessed more rapidly. Principal drivers of regional market growth are the presence of favorable public laws and programs of finance combined with elevated market penetrations. In addition, a wide range of clinical trials is anticipated to foster further market growth with the aim of boosting the efficiency and development of fresh products in the region. Due to the big existence of unsatisfactory patient requirements and the subsequent increase, Asia Pacific became the fastest growing region. In addition, a positive public initiative is anticipated to stimulate market growth in Japan and Australia throughout the forecast period. In Latin America and the MEA areas, there will be an expected increase in the number of biosimilar and generic immunosuppressants.
Scleroderma Therapeutics Market Players
Some of the top scleroderma therapeutics market companies offered in the professional report include Active Biotech AB, Daval International Ltd., BioLineRx, Ltd., Corbus pharmaceuticals, Inc., F. Hoffmann-La Roche Ltd., GlaxoSmithKline plc, Angion Biomedica Corp., Fibrocell Science, Inc., Bayer AG, Bristol-Myers Squibb Company, Allergan, Inc., and Dynavax Technologies Corporation.
Frequently Asked Questions
What is the size of global scleroderma therapeutics market in 2021?
The estimated value of global scleroderma therapeutics market in 2021 was accounted to be USD 2,912 Million.
What is the CAGR of global scleroderma therapeutics market during forecast period of 2022 to 2030?
The projected CAGR scleroderma therapeutics market during the analysis period of 2022 to 2030 is 6.4%.
Which are the key players operating in the market?
The prominent players of the global scleroderma therapeutics market are Active Biotech AB, Daval International Ltd., BioLineRx, Ltd., Corbus pharmaceuticals, Inc., F. Hoffmann-La Roche Ltd., GlaxoSmithKline plc, Angion Biomedica Corp., Fibrocell Science, Inc., Bayer AG, Bristol-Myers Squibb Company, Allergan, Inc., and Dynavax Technologies Corporation.
Which region held the dominating position in the global scleroderma therapeutics market?
North America held the dominating scleroderma therapeutics during the analysis period of 2022 to 2030.
Which region registered the fastest growing CAGR for the forecast period of 2022 to 2030?
Asia-Pacific region exhibited fastest growing CAGR for scleroderma therapeutics during the analysis period of 2022 to 2030.
What are the current trends and dynamics in the global scleroderma therapeutics market?
Growing incidence of scleroderma, and increasing pharmaceutical companies' focus on developing novel drugs drives the growth of global scleroderma therapeutics market.
By indication segment, which sub-segment held the maximum share?
Based on indication, systemic segment is expected to hold the maximum share scleroderma therapeutics market.



