Recombinant Plasma Protein Therapeutics Market | Acumen Research and Consulting
Recombinant Plasma Protein Therapeutics Market Size - Global Industry, Share, Analysis, Trends and Forecast 2024 - 2032
Published :
Report ID:
Pages :
Format :
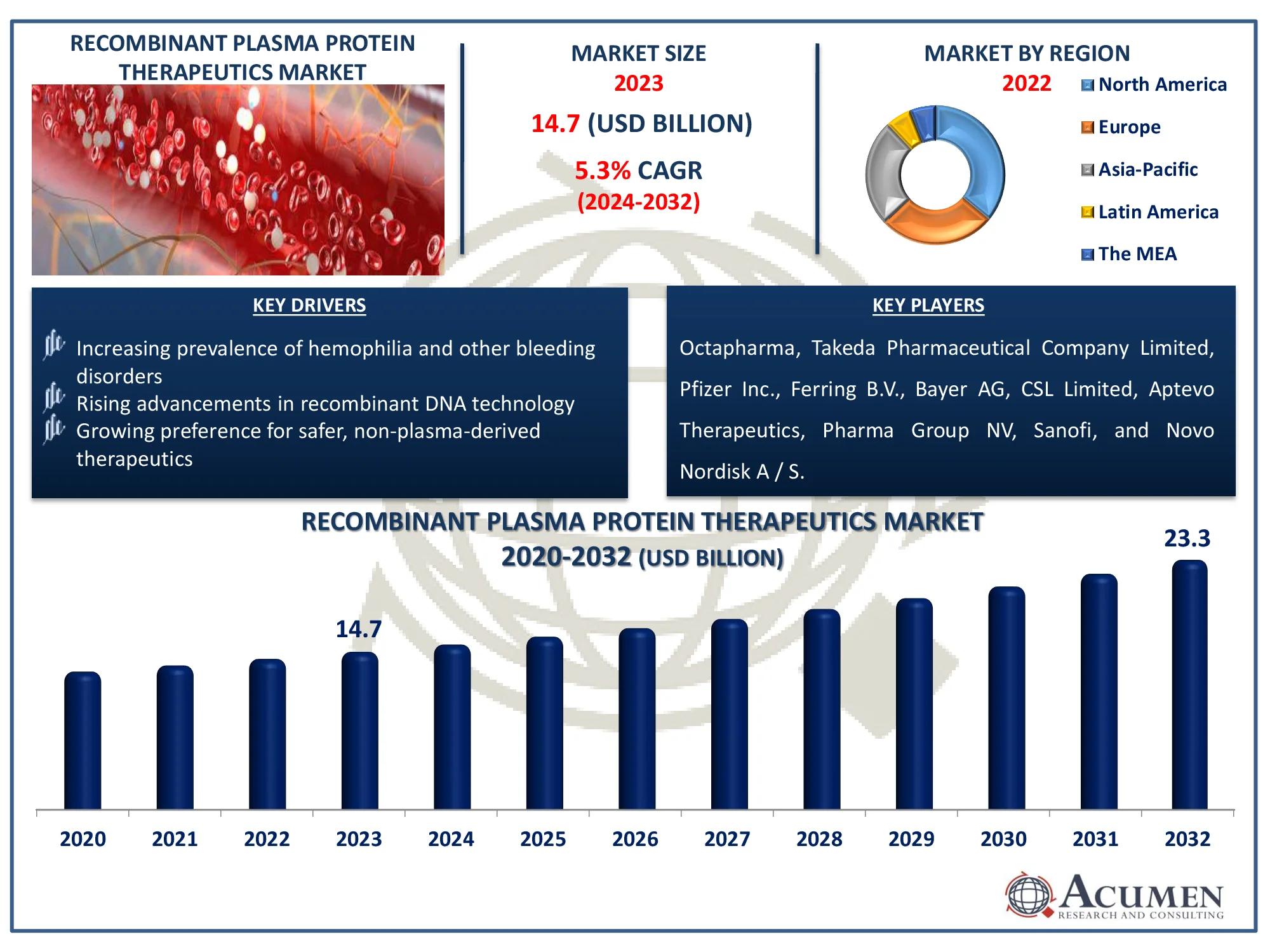
The Global Recombinant Plasma Protein Therapeutics Market Size accounted for USD 14.7 Million in 2023 and is estimated to achieve a market size of USD 23.3 Million by 2032 growing at a CAGR of 5.3% from 2024 to 2032.
Recombinant Plasma Protein Therapeutics Market (By Drug Class: Recombinant Coagulation Factors, Human C1 Esterase Inhibitor; By Cell Line: Chinese Hamster Ovary (CHO) Cell Line, Baby Hamster Kidney (BHK) Cell Line, Human Embryonic Kidney (HEK) Cell Line, Others; By Indication: Hemophilia A, Hemophilia B, Von Willebrand Disease, and Others and By Region: North America, Europe, Asia-Pacific, Latin America, and MEA)
Recombinant Plasma Protein Therapeutics Market Highlights
- The global recombinant plasma protein therapeutics market is anticipated to reach USD 23.3 million by 2032, with a projected CAGR of 5.3% between 2024 and 2032
- In 2023, the North American recombinant plasma protein therapeutics market was valued at around USD 5.3 billion
- The Asia-Pacific region is forecasted to grow at a CAGR exceeding 6.2% from 2024 to 2032
- The recombinant coagulation factors drug class held a substantial market share in 2023
- The Chinese hamster ovary (CHO) cell line sub-segment captured a notable share of the market in 2023
- Expansion of production capabilities with engineered cell lines like CHO cells is the recombinant plasma protein therapeutics market trend that fuels the industry demand
Recombinant plasma protein treatments are biopharmaceuticals created from genetically modified cells that synthesize human proteins, which have considerable advantages over typical plasma-derived medicines. These therapies are generally used to treat bleeding disorders, such as hemophilia A and B, by supplying patients with necessary clotting agents. They also help manage immunodeficiency problems by providing immunoglobulins. The introduction of recombinant technologies has improved the safety and efficacy of these treatments, lowering the risk of infections caused by blood products. These treatments are also used in surgical settings to avoid excessive bleeding and in critical care for patients who require quick attention.
Global Recombinant Plasma Protein Therapeutics Market Dynamics
Market Drivers
- Rising advancements in recombinant DNA technology
- Increasing prevalence of hemophilia and other bleeding disorders
- Growing preference for safer, non-plasma-derived therapeutics
Market Restraints
- High costs associated with recombinant protein therapies
- Limited access to advanced healthcare in developing regions
- Stringent regulatory approval processes
Market Opportunities
- Expansion into emerging markets with rising healthcare infrastructure
- Development of innovative therapies for rare blood disorders
- Strategic collaborations and partnerships for R&D advancements
Recombinant Plasma Protein Therapeutics Market Report Coverage
| Market | Recombinant Plasma Protein Therapeutics Market |
| Recombinant Plasma Protein Therapeutics Market Size 2022 |
USD 14.7 Million |
| Recombinant Plasma Protein Therapeutics Market Forecast 2032 | USD 23.3 Million |
| Recombinant Plasma Protein Therapeutics Market CAGR During 2023 - 2032 | 5.3% |
| Recombinant Plasma Protein Therapeutics Market Analysis Period | 2020 - 2032 |
| Recombinant Plasma Protein Therapeutics Market Base Year |
2022 |
| Stand-up Pouches Market Forecast Data | 2023 - 2032 |
| Segments Covered | By Drug Class, By Cell Line, By Indication, And By Geography |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
| Key Companies Profiled | Octapharma, Takeda Pharmaceutical Company Limited, Pfizer Inc., Ferring B.V., Bayer AG, CSL Limited, Aptevo Therapeutics, Pharma Group NV, Sanofi, and Novo Nordisk A / S. |
| Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Covid-19 Analysis, Regulation Analysis |
Recombinant Plasma Protein Therapeutics Market Insights
The global recombinant plasma protein treatment market is being driven by a variety of factors, including a transition from plasma-based protein use to recombinant therapy, increased awareness of rare illness care, and a greater emphasis on rare disease funding. Furthermore, a continuing increase will result in a substantial market trend in patients with uncommon hematological diseases during the forecast period, as well as product approval from authorities such as the FDA, the European Commission, and the Ministry of Health, Labor, and Welfare (Japan).
The high expense of treatment, as well as alternative treatments, is anticipated to limit market growth. On the other hand, the plasma protein therapies sector has developed profitably over the last decade, with immunoglobulin accounting for the largest part of the market.
The rising prevalence of diseases, geriatrics, and improved medicine availability in emerging economies are driving up demand for plasma products. For instance, according to National Library of Medicine, as the population ages, the worldwide cardiovascular disease burden will continue to rise, especially among older persons. New plasma-based therapeutic approvals are projected to boost demand even more. Developing countries like China and Brazil provide new investment opportunities for global market leaders.
To expand market penetration of plasma proteins, new solutions with clinical benefits such as improved efficacy and easier sample plasma extraction techniques are expected. In Asia-Pacific, significant demand for albumins and increased use of immunoglobulin are expected to drive the market throughout the projection period.
The number of regulatory approvals for patients with uncommon diseases such as hemophilia and other bleeding disorders has increased during the last three years. These approvals enabled businesses to grow and benefit from their product portfolios.
Recombinant Plasma Protein Therapeutics Market Segmentation
The worldwide market for recombinant plasma protein therapeutics is split based on drug class, cell line, indication, and geography.
Recombinant Plasma Protein Therapeutics Drug Class
- Recombinant Coagulation Factors
- Recombinant Coagulation Factor VIII
- Recombinant Coagulation Factor IX
- Recombinant Coagulation Factor VIIa
- Others
- Human C1 Esterase Inhibitor
According to the recombinant plasma protein therapeutics industry analysis, because of their importance in treating hemophilia and other bleeding disorders, ombinant coagulation factors are projected to dominate the market. Among them, Recombinant Coagulation Factor VIII is experiencing rapid expansion, owing to its success in treating hemophilia A, the most common type of hemophilia. Advances in recombinant DNA technology have increased the safety and reliability of Factor VIII therapy, lowering the danger of blood-borne infection. Additionally, rising awareness and diagnosis of hemophilia are driving up global demand.
Recombinant Plasma Protein Therapeutics Cell Line
- Chinese Hamster Ovary (CHO) Cell Line
- Baby Hamster Kidney (BHK) Cell Line
- Human Embryonic Kidney (HEK) Cell Line
- Others
In terms of cell lines, the cell-line section of Chinese hamster ovary (CHO) is predicted to grow at a rapid rate due to the rising utilization of CHO cell lines for medicinal applications. CHO cells produce high protein expression yields and are preferred for their scalability in industrial biomanufacturing processes. Their ability to synthesize complex proteins with human-like glycosylation improves the safety and efficacy of therapies. Furthermore, ongoing developments in CHO cell engineering are enhancing productivity and lowering production costs, which is supporting their growing market use.
Recombinant Plasma Protein Therapeutics Indication
- Hemophilia A
- Hemophilia B
- Von Willebrand Disease
- Others
According to the recombinant plasma protein therapeutics market forecast, hemophilia A is experiencing strong growth in the market as recombinant medicines, particularly Recombinant Factor VIII, become more widely used. These therapies provide a safer alternative to plasma-derived treatments by lowering the danger of viral transmission and immunological responses. Advances in biotechnology, together with growing knowledge of hemophilia diagnosis, are driving demand for more effective and dependable treatment alternatives. Furthermore, continued research and development in extended half-life treatments improves patient outcomes, driving further market growth.
Recombinant Plasma Protein Therapeutics Market Regional Outlook
North America
- U.S.
- Canada
Europe
- U.K.
- Germany
- France
- Spain
- Rest of Europe
Asia-Pacific
- India
- Japan
- China
- Australia
- South Korea
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Rest of Latin America
The Middle East & Africa
- South Africa
- GCC Countries
- Rest of the Middle East & Africa (ME&A)
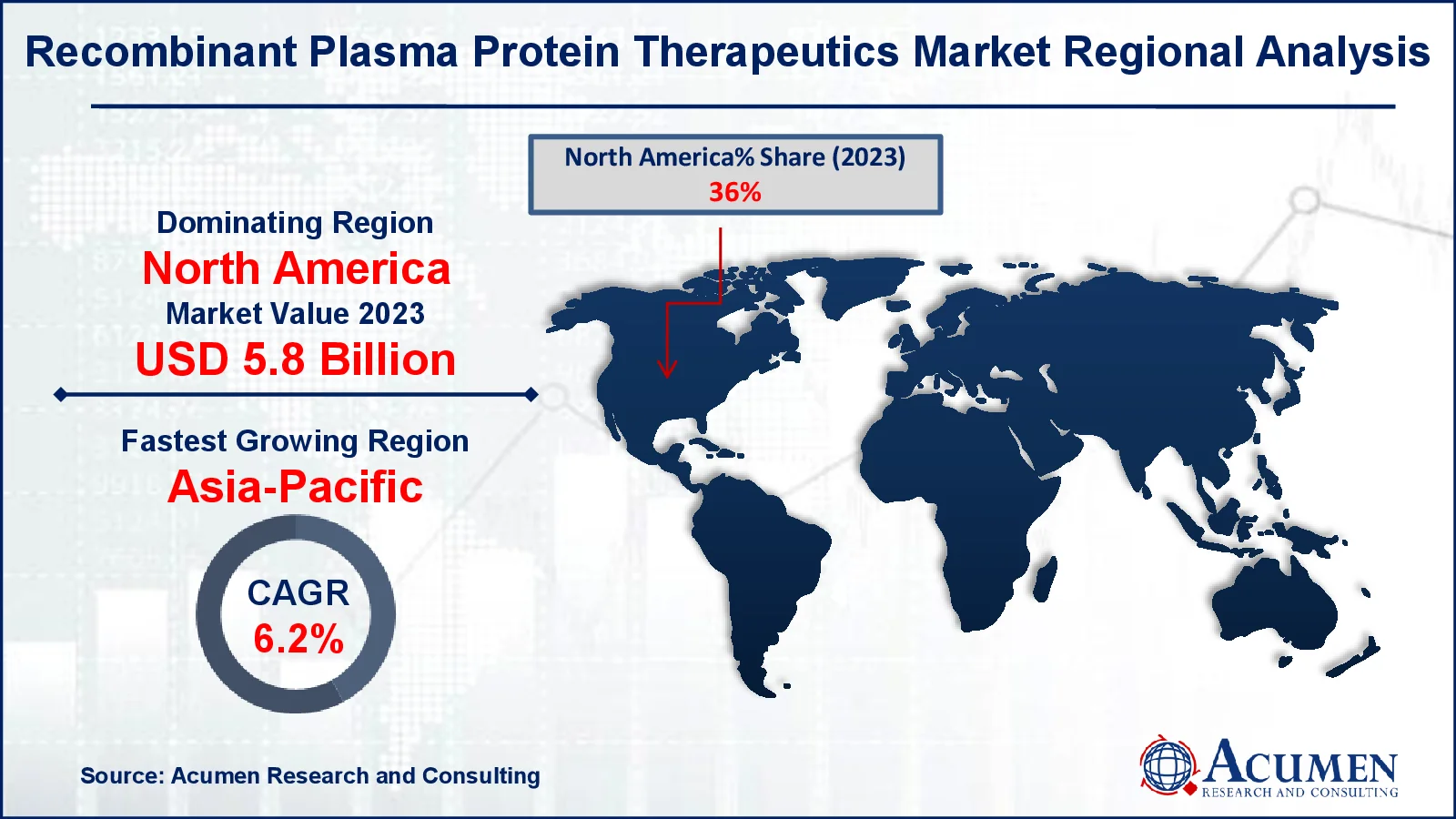
Recombinant Plasma Protein Therapeutics Market Regional Analysis
For several reasons, North America is likely to command a sizable worldwide market share. As demand for recombinant coagulation factor VII and IX rises, the US market will expand rapidly, with an increasing number of market players offering recombinant plasma proteins and a greater emphasis on the management of rare diseases such as hemophilia A, hamophilia B, and von Willebrand disease.
The Asia-Pacific region is seeing considerable expansion in the recombinant plasma protein therapeutics market as healthcare investments increase, access to new medical technology improves, and awareness of hemophilia and other blood disorders spreads. Economic growth and rising healthcare infrastructure in nations such as China and India are fueling demand for novel therapies.
In Europe, a rising number of established biotechnology and pharmaceutical businesses, as well as research institutes, universities, and industry players, have secured financing for research and development investigations into recombinant DNA technology, with the goal of expanding the market. Furthermore, developments in genetic engineering and biotechnology have considerably accelerated the creation of novel illness therapeutic items in countries such as Germany and the United Kingdom and France.
Recombinant Plasma Protein Therapeutics Market Players
Some of the top recombinant plasma protein therapeutics companies offered in our report includes Octapharma, Takeda Pharmaceutical Company Limited, Pfizer Inc., Ferring B.V., Bayer AG, CSL Limited, Aptevo Therapeutics, Pharma Group NV, Sanofi, and Novo Nordisk A/S.
Frequently Asked Questions
How big is the recombinant plasma protein therapeutics market?
The recombinant plasma protein therapeutics market size was valued at USD 14.7 billion in 2023.
What is the CAGR of the global recombinant plasma protein therapeutics market from 2024 to 2032?
The CAGR of recombinant plasma protein therapeutics is 5.3% during the analysis period of 2024 to 2032.
Which are the key players in the recombinant plasma protein therapeutics market?
The key players operating in the global market are including Octapharma, Takeda Pharmaceutical Company Limited, Pfizer Inc., Ferring B.V., Bayer AG, CSL Limited, Aptevo Therapeutics, Pharma Group NV, Sanofi, and Novo Nordisk A / S.
Which region dominated the global recombinant plasma protein therapeutics market share?
North America held the dominating position in recombinant plasma protein therapeutics industry during the analysis period of 2024 to 2032.
Which region registered fastest CAGR from 2024 to 2032?
Asia-Pacific region exhibited fastest growing CAGR for market of recombinant plasma protein therapeutics during the analysis period of 2024 to 2032.
What are the current trends and dynamics in the global recombinant plasma protein therapeutics industry?
The current trends and dynamics in the recombinant plasma protein therapeutics industry include increasing prevalence of hemophilia and other bleeding disorders, rising advancements in recombinant DNA technology, and growing preference for safer, non-plasma-derived therapeutics.
Which drug class held the maximum share in 2023?
The recombinant coagulation factors drug class held the maximum share of the recombinant plasma protein therapeutics industry. 



