Pharmacovigilance Market Size - Global Industry, Share, Analysis, Trends and Forecast 2023 - 2032
Published :
Report ID:
Pages :
Format :
Pharmacovigilance Market Size - Global Industry, Share, Analysis, Trends and Forecast 2023 - 2032
Report Coverage
- Industry Dynamics
- Market Size and Forecast Data
- Segment Analysis
- Competitive Landscape
- Regional Analysis with a Niche Focus on Country-Level Data
- High Level Analysis - Porter's, PESTEL, Value Chain, etc.
- Company Profiles of Key Players
- Option to Customize the Report As Per Your Specific Need
Request Sample Report
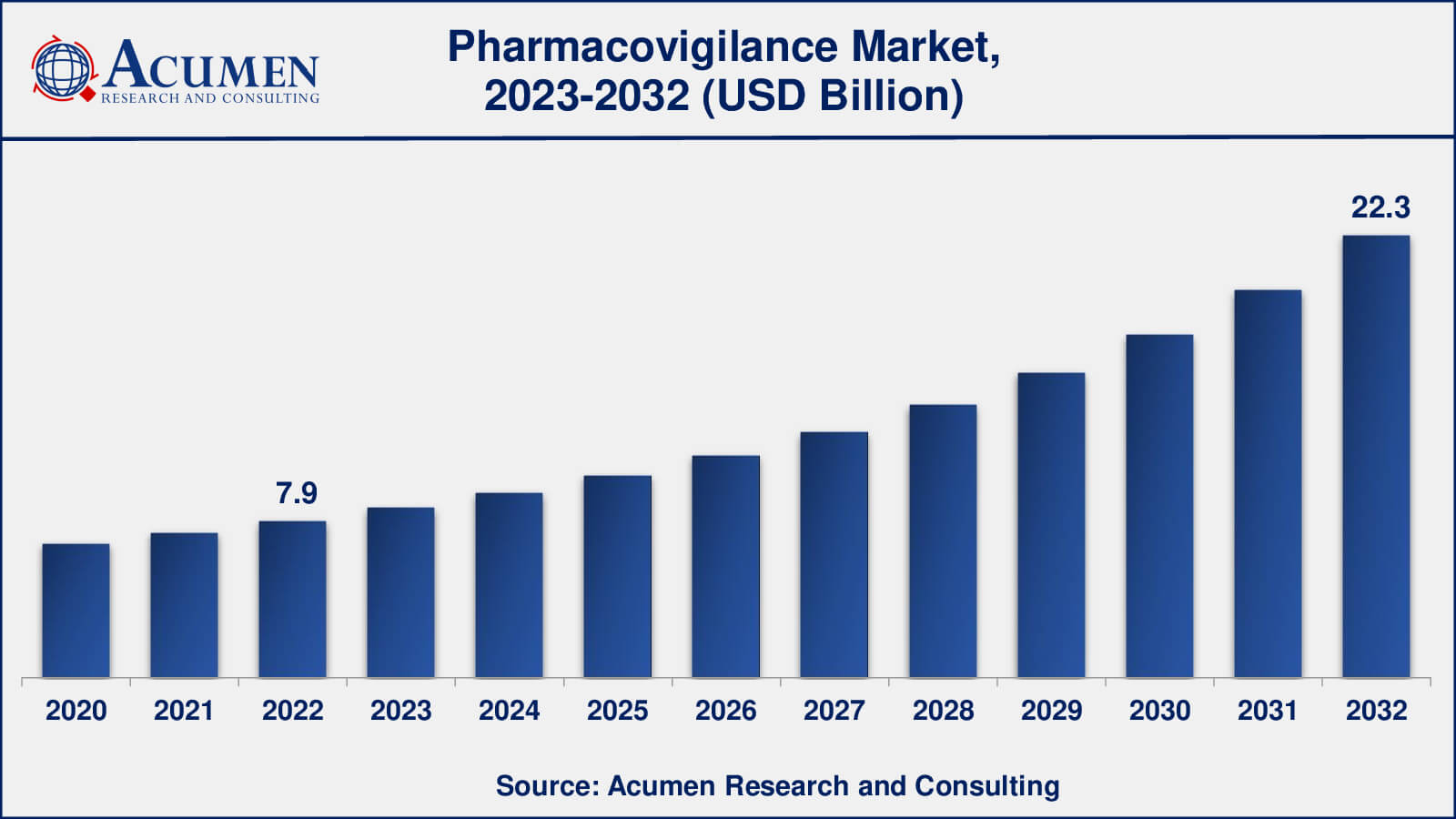
The global Pharmacovigilance Market gathered USD 7.9 Billion in 2022 and is expected to reach USD 22.3 Billion by 2032, growing at a CAGR of 11.2% from 2023 to 2032.
Pharmacovigilance Market Highlights
- The global pharmacovigilance market is poised to achieve a revenue of USD 22.3 billion by 2032, experiencing a CAGR of 11.2% from 2023 to 2032
- In 2022, the North America pharmacovigilance market held a value of approximately USD 2.4 billion
- The Asia-Pacific pharmacovigilance market is expected to witness substantial growth, with a projected CAGR of over 12% from 2023 to 2032
- Among clinical trial phases, the phase IV sub-segment accounted for revenue exceeding USD 6 billion in 2022
- In terms of service, the contract outsourcing sub-segment claimed a significant share, surpassing 60% in 2022
- Expansion of pharmacovigilance services in Asia-Pacific is one of the notable pharmacovigilance market trends

Pharmacovigilance is a study focused on the safety and efficacy of medical products or drugs. It involves the collection, detection, evaluation, and prevention of adverse effects related to these Services. Pharmacovigilance plays a crucial role in analyzing the side effects of drugs and assessing their impact. It encompasses various phases of the drug lifecycle, including drug discovery and development, preclinical research, clinical research, and post-marketing surveillance. The prevalence of adverse drug reactions and stringent safety regulations enforced by government regulatory agencies are expected to drive the growth of the pharmacovigilance market during the forecast period.
Global Pharmacovigilance Market Dynamics
Market Drivers
- Increasing global pharmaceutical and biotechnology R&D activities
- Growing awareness of drug safety and regulatory compliance
- Rising incidence of adverse drug reactions (ADRs)
- Expanding use of pharmacovigilance in post-marketing surveillance
Market Restraints
- High implementation and maintenance costs of pharmacovigilance systems
- Complex and evolving regulatory requirements
- Data privacy and security concerns
- Shortage of skilled pharmacovigilance professionals
Market Opportunities
- Outsourcing of pharmacovigilance services
- Integration of real-world data and electronic health records
- Use of blockchain technology for data integrity
Pharmacovigilance Market Report Coverage
| Market | Pharmacovigilance Market |
| Pharmacovigilance Market Size 2022 | USD 7.9 Billion |
| Pharmacovigilance Market Forecast 2032 | USD 22.3 Billion |
| Pharmacovigilance Market CAGR During 2023 - 2032 | 11.2% |
| Pharmacovigilance Market Analysis Period | 2020 - 2032 |
| Pharmacovigilance Market Base Year |
2022 |
| Pharmacovigilance Market Forecast Data | 2023 - 2032 |
| Segments Covered | By Clinical Trial Phase, By Service, By Type, By Therapeutic Area, And By Geography |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
| Key Companies Profiled | ArisGlobal, BioClinica Inc., Capgemini, Cognizant, ICON plc., IQVIA, Laboratory Corporation of America Holdings, Parexel International Corp., TAKE Solutions Ltd., United BioSource LLC, Wipro Ltd., and ClinQuest Group B.V. (Linical Americas). |
| Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Covid-19 Analysis, Regulation Analysis |
Pharmacovigilance Market Insights
Stringent regulations imposed by various drug approval agencies, a rising incidence of adverse drug reactions (ADRs), and heightened patient awareness regarding drug safety are the primary drivers of growth in the pharmacovigilance market. Additionally, the market is propelled by rigorous guidelines governing clinical trials for new medication therapies and stringent requirements for electronic drug record-keeping. Leading pharmaceutical and biotechnology companies are closely aligning with prominent regulatory bodies to ensure the safe production of medicines.
The recent trend of outsourcing pharmacovigilance services to contract research organizations (CROs) and business process outsourcing (BPO) firms has significantly streamlined the medication regulation system. Outsourcing has brought about enhanced operational efficiency, strict compliance with regulations, and improved strategic outcomes, thus fostering the expansion of the drug supervision market.
However, the growth of the market is hampered by concerns related to patient data security, a shortage of expertise, and the increasing online sales of drugs.
Pharmacovigilance Market Segmentation
The worldwide market for pharmacovigilance is split based on clinical trial phase, service, type, therapeutic area, end-use, and geography.
Pharmacovigilance Clinical Trial Phases
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
The market is segmented into preclinical, phase I, phase II, phase III, and phase IV based on the clinical trial phase. In 2022, phase IV, or post-marketing clinical trials, led the market. Pharmacovigilance (PV) solutions serve as additional safety measures for drugs undergoing clinical trials. Phase IV is a critical stage in clinical trials as it allows for the identification of unexpected adverse reactions to medications after they have entered the market. Consequently, the data collected and evaluated in this phase are expected to hold the greatest importance. This is attributed to extensive pharmaceutical testing on a large patient demographic once the medication is on the market.
On the other hand, Phase III is projected to exhibit substantial growth. Phase III studies aim to determine drug effectiveness and provide further information on potential drug interactions, safety, and efficacy before the drug is marketed. These factors are anticipated to contribute significantly to revenue generation in the near future.
Pharmacovigilance Services
- In-house
- Contract Outsourcing
As per the pharmacovigilance market analysis, In 2022, contract outsourcing dominated the market and is projected to experience the fastest growth in the coming years. This growth is attributed to the advantages of outsourcing, such as risk mitigation, resource flexibility, lower initial investment, and reduced fixed costs. Outsourcing contracts provide solutions such as standard operating procedures (SOPs), pharmacovigilance (PV) audits, and other customized services.
The rapid emergence of contract research organizations (CROs), particularly in emerging economies like India, China, and Japan, offering end-to-end clinical trial solutions that facilitate resource sharing, cost efficiency, resource flexibility, and operational capacity development, has contributed to the dynamic growth of the contract outsourcing segment.
Outsourcing services encompass addressing complex regulatory requirements, adding scalability to accommodate a growing product portfolio, and supporting aggressive cost objectives. PV services offer a wide range of advantages. Contract outsourcing also simplifies the complexity of clinical trials, expedites trial approvals, and optimizes internal resource utilization.
In the in-house segment, moderate growth is predicted over the forecast period, driven by extensive research and development efforts by major pharmaceutical and biotechnology companies in the development of new drugs. The industry is expected to be well-served by this trend in the coming years.
Pharmacovigilance Types
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
Based on type, the spontaneous reporting is a dominant and widely utilized method in pharmacovigilance for the detection and monitoring of adverse drug reactions (ADRs). One key reason for its dominance is its simplicity and accessibility. Healthcare professionals, including physicians and pharmacists, as well as patients themselves, can easily report suspected adverse reactions to regulatory authorities or pharmaceutical companies. This ease of reporting encourages a higher volume of reports, thereby increasing the likelihood of detecting rare or unexpected ADRs that might go unnoticed in more structured and controlled data collection methods. The spontaneity of this approach ensures that emerging safety concerns can be rapidly identified, allowing for swift regulatory action when necessary, such as label updates or product recalls.
Pharmacovigilance Therapeutic Areas
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
According to the pharmacovigilance market forecast, oncology stands out as a dominant therapeutic area in the pharmacovigilance market due to several compelling factors. First and foremost, the prevalence of cancer is a significant driver. Cancer is a global health concern, with millions of people diagnosed with various forms of the disease each year. The sheer volume of cancer patients, along with the increasing incidence of cancer cases worldwide, necessitates extensive research, drug development, and pharmacovigilance efforts to address the unique safety challenges associated with oncology drugs.
Another key reason for oncology's dominance is the rapid pace of innovation and drug development in this field. Advances in our understanding of the molecular mechanisms underlying cancer, coupled with breakthroughs in targeted therapies and immunotherapies, have led to the introduction of numerous new oncology drugs in recent years. These innovative treatments offer hope to cancer patients but also come with complex safety profiles that require vigilant pharmacovigilance. The need to closely monitor and manage adverse events, drug interactions, and long-term effects of these therapies underscores the critical role of pharmacovigilance in oncology, making it a central focus of the pharmaceutical industry.
Pharmacovigilance End-Uses
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
According to the pharmacovigilance market analysis, pharmaceutical companies are a dominating end-use sector in the pharmacovigilance market for several compelling reasons. Firstly, the pharmaceutical industry is characterized by an extensive and diverse product portfolio, encompassing a wide range of drugs and therapeutic areas. This diversity results in a substantial volume of adverse event reports and data that necessitate comprehensive pharmacovigilance systems and processes. Pharmaceutical companies are responsible for developing, manufacturing, and distributing drugs, making them acutely aware of the importance of monitoring and ensuring the safety of their products throughout their lifecycle, from preclinical testing to post-market surveillance.
Secondly, the stringent regulatory environment surrounding pharmaceuticals further reinforces the significance of pharmacovigilance for this sector. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), require pharmaceutical companies to demonstrate the safety and efficacy of their products before approval and to continuously monitor their safety after market launch. Failure to meet these regulatory requirements can result in severe consequences, including product recalls and legal liabilities. Therefore, pharmaceutical companies invest significantly in pharmacovigilance to adhere to these regulations, maintain compliance, and protect their reputation in the market.
Pharmacovigilance Market Regional Segmentation
North America
- U.S.
- Canada
Europe
- U.K.
- Germany
- France
- Spain
- Rest of Europe
Asia-Pacific
- India
- Japan
- China
- Australia
- South Korea
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Rest of Latin America
The Middle East & Africa
- South Africa
- GCC Countries
- Rest of the Middle East & Africa (ME&A)
Pharmacovigilance Market Regional Analysis
North America held the largest revenue share in 2022, primarily due to the presence of key players in the pharmaceutical and medical products industries. The region contributed significantly to overall sales in the pharmacovigilance market. The increasing incidence of drug abuse and associated adverse drug reactions, which are leading causes of death and morbidity, are significant drivers of growth in North America. Furthermore, major pharmaceutical companies' increased investments in the development of new drugs are expected to further boost the regional market. Consequently, the number of clinical trials and post-marketing surveillance activities has increased significantly, driven by the large-scale drug production, contributing to the overall market growth.
In the projected period, Asia-Pacific is expected to register a lucrative compound annual growth rate (CAGR) of over 12 percent, owing to the presence of numerous external organizations. This is expected to stimulate the demand for pharmacovigilance in the region, fostering improved productivity, cost-efficiency, and resource sharing. Additionally, regional market growth is driven by increased patient awareness, rising investments, and government initiatives aimed at meeting the healthcare needs of the growing population.
Pharmacovigilance Market Players
Some of the top Pharmacovigilance companies offered in our report includes ArisGlobal, BioClinica Inc., Capgemini, Cognizant, ICON plc., IQVIA, Laboratory Corporation of America Holdings, Parexel International Corp., TAKE Solutions Ltd., United BioSource LLC, Wipro Ltd., and ClinQuest Group B.V. (Linical Americas).
Frequently Asked Questions
What was the size of the global pharmacovigilance market in 2022?
The size of pharmacovigilance market was USD 7.9 billion in 2022.
What is the pharmacovigilance market CAGR from 2023 to 2032?
The pharmacovigilance market CAGR during the analysis period of 2023 to 2032 is 11.2%.
Which are the key players in the pharmacovigilance market?
The key players operating in the global pharmacovigilance market are ArisGlobal, BioClinica Inc., Capgemini, Cognizant, ICON plc., IQVIA, Laboratory Corporation of America Holdings, Parexel International Corp., TAKE Solutions Ltd., United BioSource LLC, Wipro Ltd., and ClinQuest Group B.V. (Linical Americas).
Which region dominated the global pharmacovigilance market share?
North America region held the dominating position in pharmacovigilance industry during the analysis period of 2023 to 2032.
Which region registered fastest CAGR from 2023 to 2032?
Asia-Pacific region exhibited fastest growing CAGR for market of pharmacovigilance during the analysis period of 2023 to 2032.
What are the current trends in the global pharmacovigilance industry?
The current trends and dynamics in the pharmacovigilance industry include increasing global pharmaceutical and biotechnology R&D activities, growing awareness of drug safety and regulatory compliance, and rising incidence of adverse drug reactions (ADRs).
Which material held the maximum share in 2022?
The phase IV held the maximum share of the pharmacovigilance industry.?


