Monoclonal Antibodies Market Size - Global Industry, Share, Analysis, Trends and Forecast 2023 - 2032
Published :
Report ID:
Pages :
Format :
Monoclonal Antibodies Market Size - Global Industry, Share, Analysis, Trends and Forecast 2023 - 2032
Report Coverage
- Industry Dynamics
- Market Size and Forecast Data
- Segment Analysis
- Competitive Landscape
- Regional Analysis with a Niche Focus on Country-Level Data
- High Level Analysis - Porter's, PESTEL, Value Chain, etc.
- Company Profiles of Key Players
- Option to Customize the Report As Per Your Specific Need
Request Sample Report
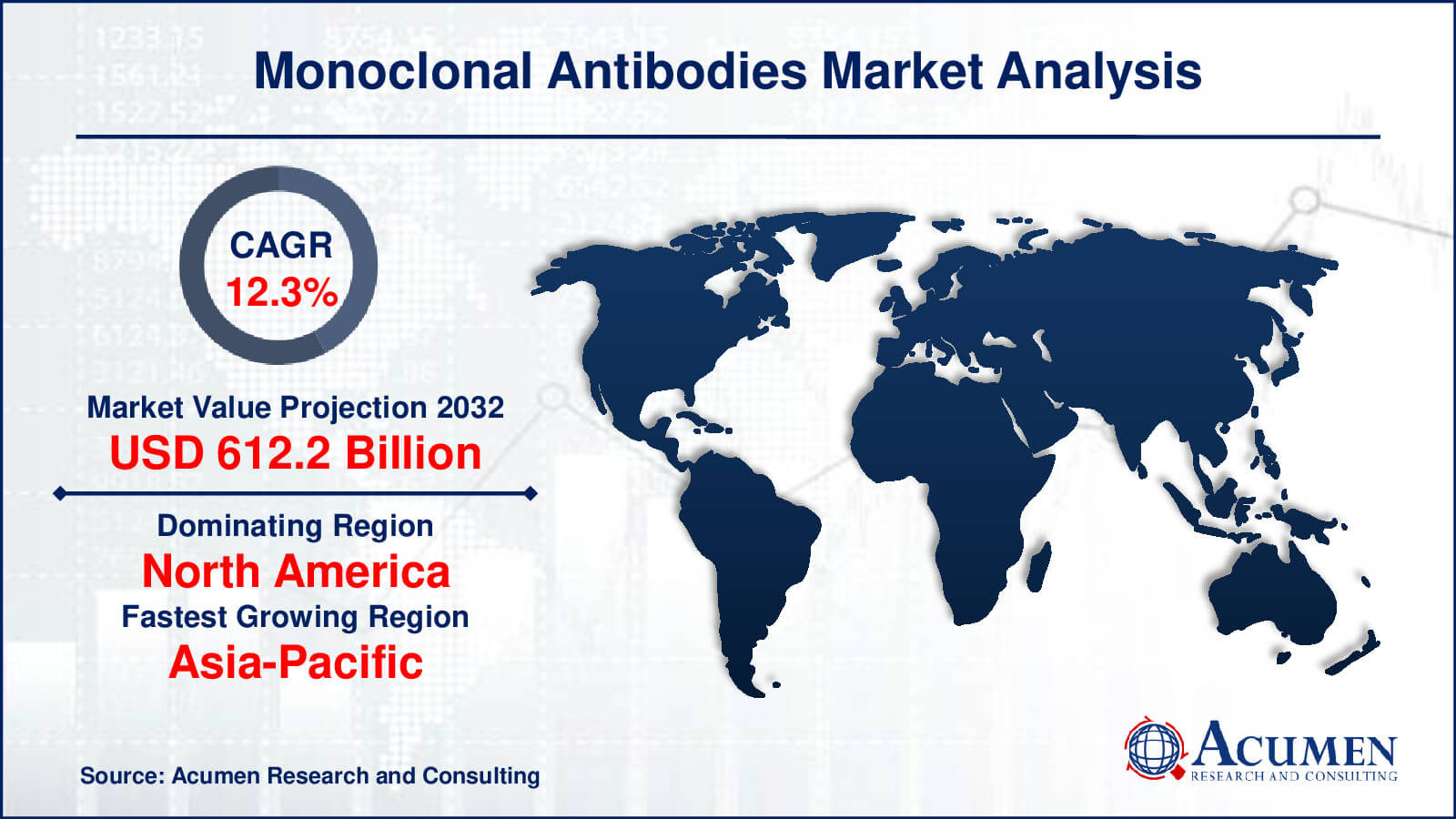
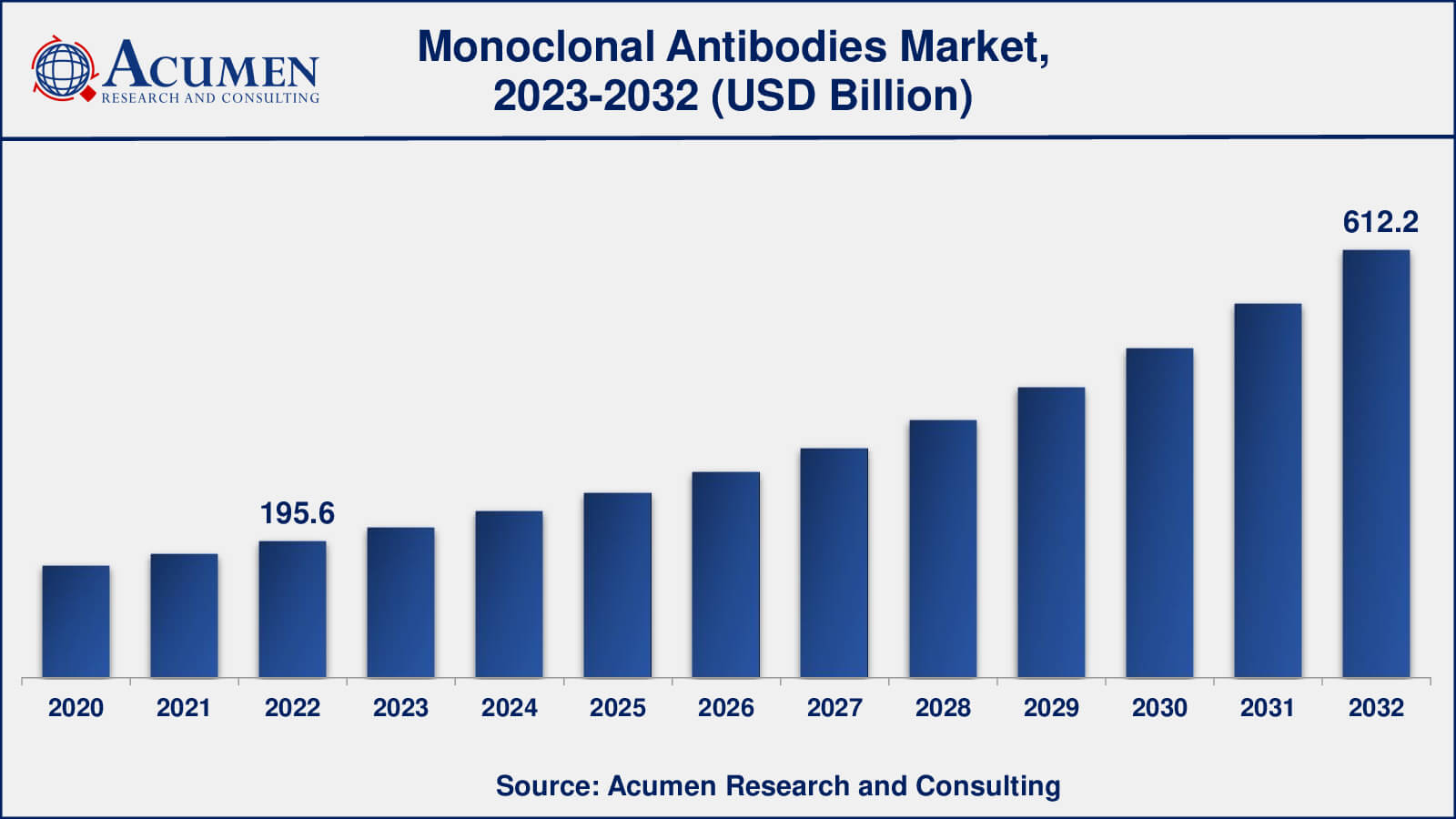
The global Monoclonal Antibodies Market size was valued at USD 195.6 Billion in 2022 and is projected to attain USD 612.2 Billion by 2032 mounting at a CAGR of 12.3% from 2023 to 2032.
Monoclonal Antibodies Market Highlights
- Global monoclonal antibodies market revenue is poised to garner USD 612.2 billion by 2032 with a CAGR of 12.3% from 2023 to 2032
- North America monoclonal antibodies market value occupied around USD 91.9 million in 2022
- Asia-Pacific monoclonal antibodies market growth will record a CAGR of more than 13% from 2023 to 2032
- Among source type, the human sub-segment occupied over US$ 99.7 billion revenue in 2022
- Based on end-use, the hospitals sub-segment gathered around 54% share in 2022
- Emerging markets and untapped therapeutic areas present growth potential is a popular monoclonal antibodies market trend that drives the industry demand
Monoclonal antibodies are laboratory-created molecules that replicate the immune system's capacity to fight off dangerous invaders. These antibodies are produced by cloning a single immune cell into identical duplicates, thus the term "monoclonal." They are a form of immunotherapy that has gained popularity because to their success in treating a variety of medical problems, including some types of cancer and autoimmune disorders. Scientists begin by immunizing a mouse or similar animal with the target antigen, which is often a protein located on the surface of the pathogen or afflicted cells. In reaction to the antigen, the animal's immune system creates a variety of antibodies. The antibodies that selectively bind to the antigen of interest are identified and separated from this pool of antibodies.
Once an antibody-producing cell is found, it is isolated and united with a cancer cell known as a myeloma cell. This union produces a hybridoma cell, which can generate identical antibodies in vast quantities indefinitely. These monoclonal antibodies can then be isolated and purified for medicinal applications.
Monoclonal antibodies have a wide range of medical uses. They can be developed to target specific proteins on the surface of cancer cells, interfering with their proliferation and signaling pathways in cancer therapy. Monoclonal antibodies can be used to limit the immune response and decrease inflammation in autoimmune illnesses, when the immune system assaults the body's own tissues.
Furthermore, monoclonal antibodies have been used in diagnostics to identify the presence of certain chemicals in a patient's blood or tissue samples, such as hormones or infectious pathogens. They've also played an important role in the development of infectious illness medicines, particularly current efforts to battle the COVID-19 pandemic. Monoclonal antibody treatments, such as those developed for COVID-19, entail injecting antibodies manufactured in a laboratory into a patient's body to target the virus and neutralize its effects.
Global Monoclonal Antibodies Market Dynamics
Market Drivers
- Growing demand for personalized and targeted therapies
- Increasing prevalence of chronic diseases, driving research into precise treatment options
- Advancements in biotechnology and genetic engineering, enhancing antibody production processes
- Rise in funding for healthcare R&D, fostering monoclonal antibody innovation
Market Restraints
- High development costs and complex manufacturing processes
- Stringent regulatory requirements for approval and safety
- Potential for immune system reactions and adverse effects
- Limited access and affordability in certain regions, impacting global adoption
Market Opportunities
- Expanding applications in oncology, autoimmune disorders, and infectious diseases
- Collaborations between pharmaceutical companies and research institutions for novel therapies
- Development of bispecific and multispecific antibodies for enhanced therapeutic effects
Monoclonal Antibodies Market Report Coverage
| Market | Monoclonal Antibodies Market |
| Monoclonal Antibodies Market Size 2022 | USD 195.6 Billion |
| Monoclonal Antibodies Market Forecast 2032 | USD 612.2 Billion |
| Monoclonal Antibodies Market CAGR During 2023 - 2032 | 12.3% |
| Monoclonal Antibodies Market Analysis Period | 2020 - 2032 |
| Monoclonal Antibodies Market Base Year | 2022 |
| Monoclonal Antibodies Market Forecast Data | 2023 - 2032 |
| Segments Covered | By Source Type, By Production Method, By Application, By End-Use, And By Geography |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
| Key Companies Profiled | Abbott Laboratories, Amgen Inc., AstraZeneca plc, Bayer AG, Biogen Inc., Bristol Myers Squibb, Daiichi Sankyo Company, Limited, Eli Lilly And Company, F. Hoffman-La Roche Ltd., GlaxoSmithKline plc, Johnson & Johnson Services, Inc., Merck & Co., Inc., Novartis AG, Pfizer Inc., and Sanofi S.A. |
| Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Covid-19 Analysis, Regulation Analysis |
Monoclonal Antibodies Market Insights
The market for monoclonal antibodies is characterized by a complex interaction of many variables that affect its growth trajectory collectively. Monoclonal antibodies have grown in popularity as a result of market forces such as rising demand for personalized and targeted medicines. These molecules provide a precise and successful way to treating a variety of ailments, harmonizing with the rising trend towards personalized treatment plans. Furthermore, the increased frequency of chronic illnesses such as cancer and autoimmune disorders has fueled research and investment in medicines that take advantage of the specificity of monoclonal antibodies.
Biotechnology and genetic engineering advancements have emerged as major drivers. These breakthroughs have transformed the manufacture of monoclonal antibodies, allowing for more efficient and cost-effective manufacturing techniques. As a result of the increased production capacity, the discovery of innovative therapeutics has been facilitated, making monoclonal antibodies a potential route for medical study and use. Furthermore, increased financing for healthcare research and development has supplied the resources needed to continue the creation and refining of monoclonal antibody therapeutics.
The market, however, is not without its difficulties. High development costs and complex production processes are key impediments. The intricacy of producing monoclonal antibodies, from discovery to large-scale manufacture, necessitates significant financial inputs. Regulatory barriers are also important. The strict standards for monoclonal antibody therapy approval and safety evaluation might result in longer development timeframes and higher prices. Concerns about possible immunological responses and harmful consequences highlight the importance of comprehensive testing and monitoring during the clinical phases.
Furthermore, while there is a global need for monoclonal antibodies, accessibility and price remain issues. In some areas, limited access to these therapies might restrict equitable healthcare delivery. To overcome these hurdles, pharmaceutical firms, governments, and healthcare organizations must work together to guarantee that these medications reach people in need across varied economic and geographic contexts.
On the other hand, the market is brimming with opportunity. Expanding applications in cancer, autoimmune illnesses, and infectious diseases have enormous promise. Collaborations between pharmaceutical behemoths and academic institutes stimulate innovation, which has the potential to generate game-changing cures. The creation of bispecific and multispecific antibodies that can target numerous antigens at the same time opens up new avenues for improved therapeutic effectiveness. Moreover, as the demand for innovative therapies grows, emerging markets provide unexplored development opportunities.
Finally, the monoclonal antibodies market is impacted by a plethora of elements that define its destiny collectively. While factors such as personalized medicines and biotechnological developments fuel its expansion, constraints like as regulatory regulations and restricted accessibility provide roadblocks. Opportunities in increasing applications and innovative antibody forms, on the other hand, point to a promising future based on cooperation, innovation, and a dedication to improve global healthcare outcomes.
Monoclonal Antibodies Market Segmentation
The worldwide market for monoclonal antibodies is split based on source type, production method, application, end-use, and geography.
Monoclonal Antibodies Source Types
- Murine
- Chimeric
- Humanized
- Human
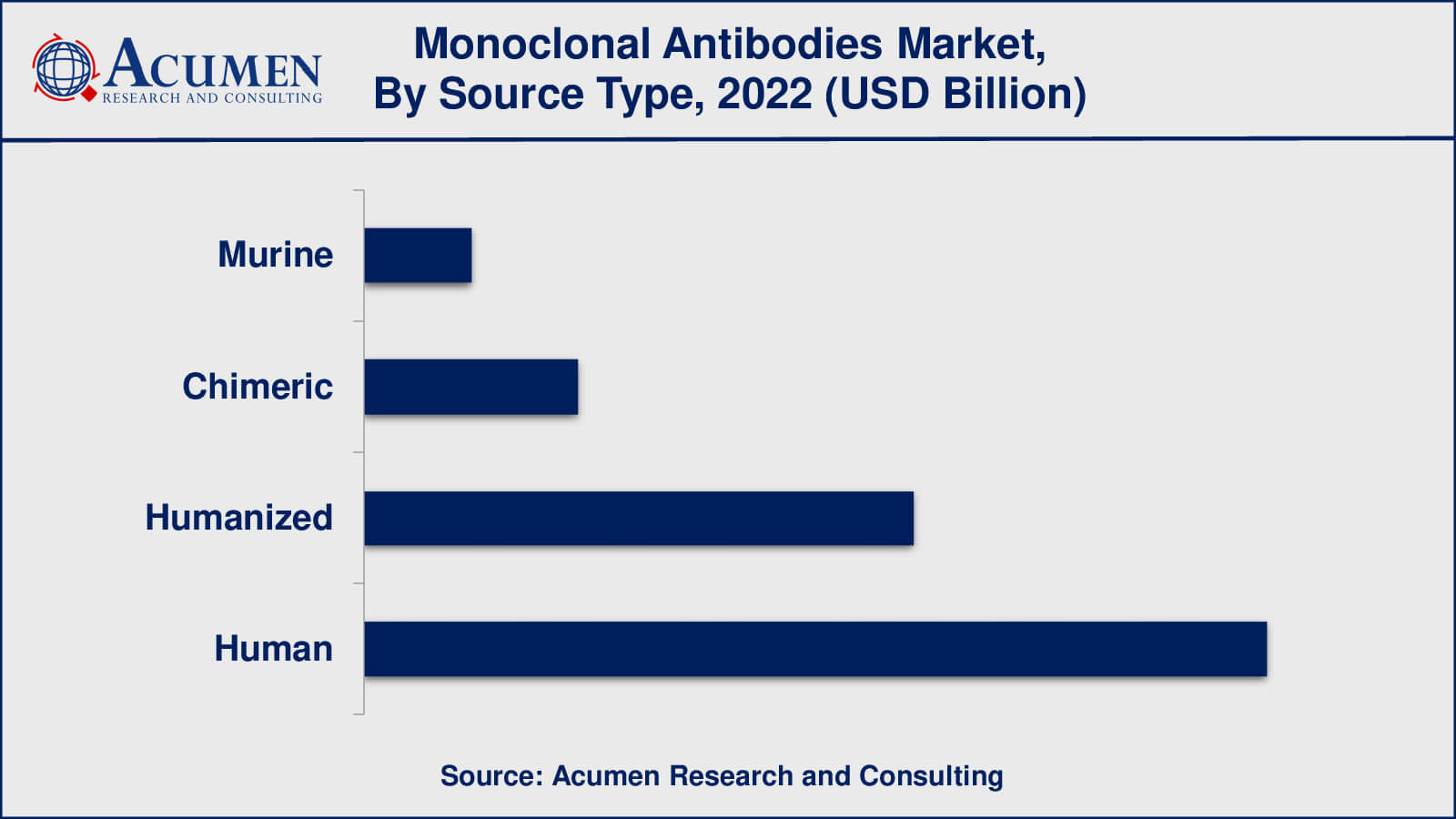
As per the monoclonal antibodies industry analysis, the trend has been changing towards humanised and completely human monoclonal antibodies, with a diminishing dependence on murine (mouse-derived) antibodies. The necessity to decrease possible immunological reactions in patients and increase antibody efficacy has prompted this move.
Murine antibodies were among the first to be produced, but their therapeutic use has been restricted due to their ability to elicit immunological responses in humans. Chimeric antibodies, which mix mouse and human components, have been produced to help reduce this immunological reaction.
Humanised antibodies, which preserve mouse antibody antigen-binding domains while adding human framework elements, were a significant breakthrough. The goal of this design was to keep the antibody's specificity while minimising possible immunogenicity. This method resulted in better clinical outcomes and fewer side effects.
Furthermore, the most human-like antibodies are entirely human monoclonal antibodies created utilising diverse methods such as phage display or transgenic mice. Because these antibodies are less immunogenic, they are intriguing candidates for therapeutic uses. The industry's efforts to improve the safety and efficacy of monoclonal antibody therapeutics are reflected in the move towards humanised and completely human antibodies.
Monoclonal Antibodies Production Method
- In Vivo
- In Vitro
In the manufacturing of monoclonal antibodies, both in vivo and in vitro approaches have been used, however in vitro methods have grown increasingly prominent over time. In vitro techniques include growing cells outside of a live organism, generally in bioreactors, allowing for regulated and scalable antibody synthesis. This method has gained popularity due to its advantages in terms of repeatability, efficiency, and reduced contamination risk.
In vivo methods, on the other hand, involve the use of animals, often mice, to generate monoclonal antibodies. This process typically involves immunizing the animal with the target antigen and then harvesting the antibody-producing cells. While this method was historically essential for generating early monoclonal antibodies, it has become less prevalent due to ethical concerns, limitations in scalability, and the availability of more advanced in vitro techniques.
Monoclonal Antibodies Applications
- Oncology
- Autoimmune Diseases
- Infectious Diseases
- Neurological Diseases
- Others
According to the monoclonal antibodies market analysis, oncology has traditionally been one of the monoclonal antibodies market's leading applications. By targeting particular chemicals on the surface of cancer cells, interfering with their growth and survival processes, and improving the immune system's capacity to recognise and fight malignant cells, monoclonal antibodies have played an important role in cancer treatment. Trastuzumab (Herceptin), rituximab (Rituxan), and bevacizumab (Avastin) are three examples of medications that have been used to treat various forms of cancer.
Monoclonal antibodies have also been used to treat autoimmune illnesses. The immune system attacks the body's own tissues in certain disorders, resulting in persistent inflammation and tissue damage. Monoclonal antibodies can be tailored to target specific immune cells or molecules implicated in the autoimmune response, assisting in immunological modulation and reducing disease severity. Adalimumab (Humira) and infliximab (Remicade) have been shown to be effective in treating autoimmune diseases such as rheumatoid arthritis and Crohn's disease.
Monoclonal Antibodies End-Uses
- Hospitals
- Specialty Centers
- Others
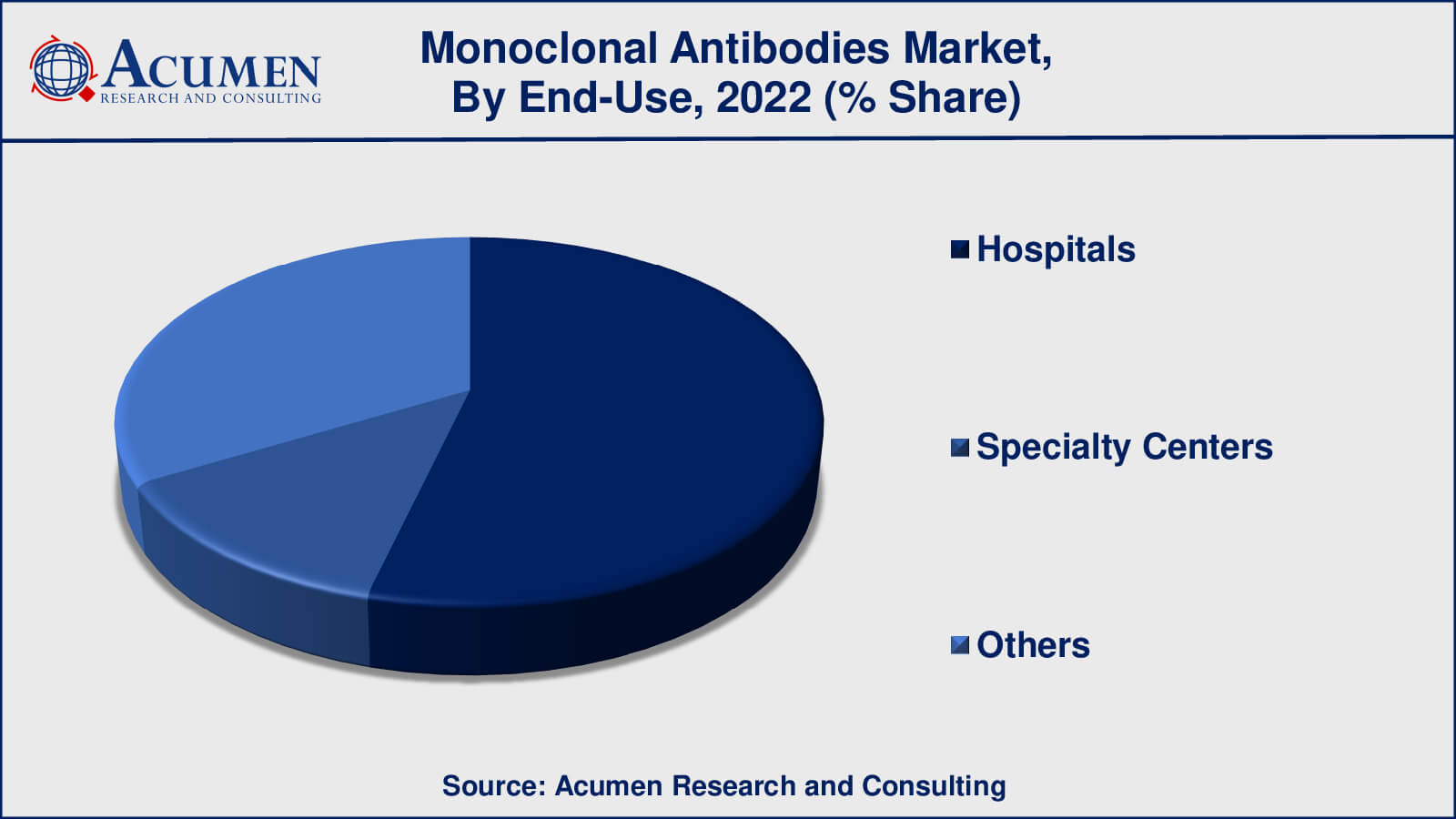
According to the monoclonal antibodies market forecast, hospitals have historically led the monoclonal antibodies market's end-use sector. Monoclonal antibody treatments are frequently provided in hospitals, where patients get specialised medical care and treatment for a variety of illnesses. Hospitals offer the infrastructure, medical knowledge, and resources needed for monoclonal antibody therapy administration and monitoring.
Speciality centres, such as specialised clinics and treatment centres, are also important in the use of monoclonal antibody therapy. These centres, such as cancer treatment centres or autoimmune disease clinics, specialise on certain medical illnesses or patient groups. They are well-equipped to offer focused and personalised care, which is consistent with the nature of monoclonal antibody therapy.
The others category contains a variety of additional scenarios where monoclonal antibody therapy may be used. Depending on the exact context and type of the therapy, this might include outpatient clinics, ambulatory care centers, and home healthcare settings.
Monoclonal Antibodies Market Regional Segmentation
North America
- U.S.
- Canada
Europe
- U.K.
- Germany
- France
- Spain
- Rest of Europe
Asia-Pacific
- India
- Japan
- China
- Australia
- South Korea
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Rest of Latin America
The Middle East & Africa
- South Africa
- GCC Countries
- Rest of the Middle East & Africa (ME&A)
Monoclonal Antibodies Market Regional Analysis
North America has long been a prominent player in the monoclonal antibody business, particularly the United States and Canada. The availability of modern healthcare systems, research institutes, and pharmaceutical businesses has aided in the development and implementation of monoclonal antibody therapy. Significant expenditures in research and development have occurred in the region, fueling innovation in therapeutic applications, notably in cancer and autoimmune illnesses.
The European Union has a solid healthcare foundation and regulatory structure, which is promoting the expansion of the monoclonal antibody industry. Germany, the United Kingdom, and France have been at the forefront of research and clinical trials. The European Medicines Agency (EMA) has been critical in approving monoclonal antibody medicines, hence increasing their availability across Europe.
The Asia-Pacific area has emerged as a fast developing market for monoclonal antibodies, headed by nations such as China, Japan, and India. Increasing healthcare spending, the incidence of chronic illnesses, and a growing desire for innovative medicines have all contributed to the widespread use of monoclonal antibody therapy. Furthermore, the region's significance in biotechnology and pharmaceutical manufacture has helped to boost local production and distribution.
Monoclonal Antibodies Market Players
Some of the top monoclonal antibodies companies offered in our report include Abbott Laboratories, Amgen Inc., AstraZeneca plc, Bayer AG, Biogen Inc., Bristol Myers Squibb, Daiichi Sankyo Company, Limited, Eli Lilly And Company, F. Hoffman-La Roche Ltd., GlaxoSmithKline plc, Johnson & Johnson Services, Inc., Merck & Co., Inc., Novartis AG, Pfizer Inc., and Sanofi S.A.
Frequently Asked Questions
What was the size of the global monoclonal antibodies market in 2022?
The size of monoclonal antibodies market was USD 195.6 billion in 2022.
What is the monoclonal antibodies market CAGR from 2023 to 2032?
The monoclonal antibodies market CAGR during the analysis period of 2023 to 2032 is 12.3%.
Which are the key players in the monoclonal antibodies market?
The key players operating in the global monoclonal antibodies market are Abbott Laboratories, Amgen Inc., AstraZeneca plc, Bayer AG, Biogen Inc., Bristol Myers Squibb, Daiichi Sankyo Company, Limited, Eli Lilly And Company, F. Hoffman-La Roche Ltd., GlaxoSmithKline plc, Johnson & Johnson Services, Inc., Merck & Co., Inc., Novartis AG, Pfizer Inc., and Sanofi S.A.
Which region dominated the global monoclonal antibodies market share?
North America region held the dominating position in monoclonal antibodies industry during the analysis period of 2023 to 2032.
Which region registered fastest CAGR from 2023 to 2032?
Asia-Pacific region exhibited fastest growing CAGR for market of monoclonal antibodies during the analysis period of 2023 to 2032.
What are the current trends in the global monoclonal antibodies industry?
The current trends and dynamics in the monoclonal antibodies industry include growing demand for personalized and targeted therapies increasing prevalence of chronic diseases, driving research into precise treatment options, and advancements in biotechnology and genetic engineering, enhancing antibody production processes.
Which source type held the maximum share in 2022?
The human source type held the maximum share of the monoclonal antibodies industry.



