Mesenchymal Stem Cells Market Size - Global Industry, Share, Analysis, Trends and Forecast 2024 - 2032
Published :
Report ID:
Pages :
Format :
Mesenchymal Stem Cells Market Size - Global Industry, Share, Analysis, Trends and Forecast 2024 - 2032
Report Coverage
- Industry Dynamics
- Market Size and Forecast Data
- Segment Analysis
- Competitive Landscape
- Regional Analysis with a Niche Focus on Country-Level Data
- High Level Analysis - Porter's, PESTEL, Value Chain, etc.
- Company Profiles of Key Players
- Option to Customize the Report As Per Your Specific Need
Request Sample Report
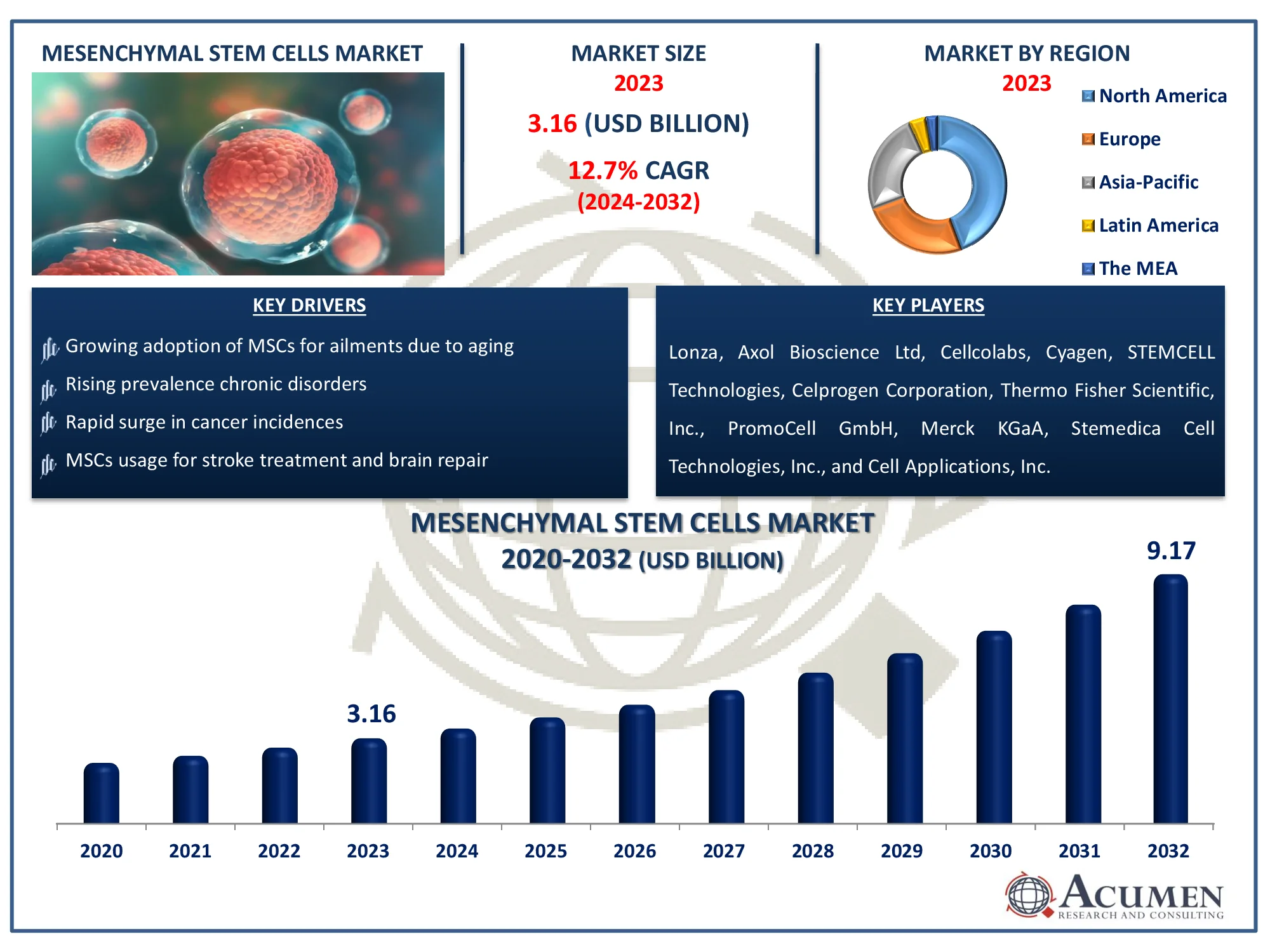
The Global Mesenchymal Stem Cells Market Size accounted for USD 3.16 Billion in 2023 and is estimated to achieve a market size of USD 9.17 Billion by 2032 growing at a CAGR of 12.7% from 2024 to 2032.
Mesenchymal Stem Cells Market Highlights
- Global mesenchymal stem cells industry revenue is poised to garner USD 9.17 billion by 2032 with a CAGR of 12.7% from 2024 to 2032
- North America mesenchymal stem cells market value occupied around USD 1.4 billion in 2023
- Asia-Pacific mesenchymal stem cells market growth will record a CAGR of more than 13.8% from 2024 to 2032
- Among products & services, the products sub-segment generated 78% of the mesenchymal stem cells market share in 2023
- Based on type, the allogenic MSCs sub-segment generated 56% mesenchymal stem cells market share in 2023
- According to the World Health Organization, chronic disease prevalence grew by 57% in the year 2020 throughout the globe
- Advancements in 3D bioprinting technology integrating MSCs for tissue engineering is the mesenchymal stem cells market trend that fuels the industry demand
The growing need for stem cell based research in regenerative medicine is a primary factor that is boosting the mesenchymal stem cells market value. In addition to that, the increasing number of research & development activities to discover new application is a very popular mesenchymal stem cells market trend that is strengthening the industry growth.
Tissue or 'adult' stem cells include mesenchymal stem cells (MSCs). They are ‘multipotent,' which means they can produce more than one type of specialized cell in the body, but not all of them. MSCs are responsible for the production of the various specialized cells found in skeletal tissues. They can differentiate or specialize into cartilage cells (chondrocytes), bone cells (osteoblasts), and fat cells, for example. Each of these specialized cells has its own distinct shape, structure, and function, and each belongs to a specific tissue.
Furthermore, mesenchymal stem cells were isolated for the first time from bone marrow and later from cord cells, adipose tissue, and molar cells. MSCs are smaller in size and more difficult to see in histological sections. Mesenchyme is a group of mesenchymal stem cells that are widely distributed throughout the body. Mesenchymal cells can divide into numerous specialized cells and grow in vitro or in vivo.
Global Mesenchymal Stem Cells Market Dynamics
Market Drivers
- Growing adoption of MSCs for ailments due to aging
- Rising prevalence chronic disorders
- Rapid surge in cancer incidences
- MSCs usage for stroke treatment and brain repair
Market Restraints
- High cost of mesenchymal stem cells therapy
- Lack of awareness regarding MSCs treatment
Market Opportunities
- Growing application of mesenchymal stem cells in regenerative medicine
- Increasing collaborations and partnership between key players
- Expansion of MSC-based clinical trials for novel therapeutic applications
Mesenchymal Stem Cells (MSCs) Market Report Coverage
|
Market |
Mesenchymal Stem Cells (MSCs) Market |
|
Mesenchymal Stem Cells Market Size 2023 |
USD 3.16 Billion |
|
Mesenchymal Stem Cells Market Forecast 2032 |
USD 9.17 Billion |
|
Mesenchymal Stem Cells Market CAGR During 2024 - 2032 |
12.7% |
|
Mesenchymal Stem Cells Market Analysis Period |
2020 - 2032 |
|
Mesenchymal Stem Cells Market Base Year |
2023 |
|
Mesenchymal Stem Cells Market Forecast Data |
2024 - 2032 |
|
Segments Covered |
By Products & Services, By Type, By Workflow Type, By Source of Isolation, By Indication, By Application, and by geography |
|
Regional Scope |
North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
|
Key Companies Profiled |
Lonza, Axol Bioscience Ltd, Cellcolabs, Cyagen, STEMCELL Technologies, Celprogen Corporation, Thermo Fisher Scientific, Inc., PromoCell GmbH, Merck KGaA, Stemedica Cell Technologies, Inc., and Cell Applications, Inc. |
|
Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Covid-19 Analysis, Regulation Analysis |
Mesenchymal Stem Cells Market Insights
Big Calls for "Bioengineering Solutions" To Fuel the Functions of Mesenchymal Stem Cells (MSCs)
According to the American Association for the Advancement of Science report, clinical trials to date show that MSCs can be safely infused in high doses and show promising results in some clinical indications. Quality control protocols to standardize MSC product potency may help reduce the risk of clinical failure, but they are unlikely to completely resolve the problem, as MSCs' innate function is not always therapeutically sufficient for disease treatment. Simple alternative bioengineering strategies that can boost the innate function of MSCs, regardless of cryopreservation, passage number, donor and tissue source, should be investigated to maximize clinical potency while preserving ease of use. Furthermore, bioengineering can be a powerful platform for translating new insights gained from a fundamental understanding of MSC behavior following infusion into more effective therapies.
Rising Prevalence of Cancer Incidences Have Gained Significant Attention for the Global Mesenchymal Stem Cells (MSCs) Market
Tumor microenvironment interacts with tumor cells, creating an environment that can either contribute to or suppress tumor development. Among the cells that play a role in the tumor microenvironment, mesenchymal stem cells (MSCs) have been shown to be capable of orchestrating the fate of tumor cells, attracting interest in the field. MSCs have been thought to have dual-bladed effects, implying either tumorigenic or anti-tumor activity. Clinical studies, on the other hand, have revealed that MSCs have a promising potential for treating human cancer cells. Among the advantageous properties of MSCs is their natural tumor-trophic migration ability, which allows for drug delivery and, thus, targeted treatment to detach tumor and metastatic cells. Furthermore, because of their easily implemented characteristics, these cells have been the target of engineering approaches in order to obtain the desired expression of anti-angiogenic, anti-proliferative, and pro-apoptotic properties, depending on the tumor type.
MSCs Possess Clinical Effects for Stroke Treatment And Brain Repair in Patient Group Bolster the Growth of the Global Market
According to the American Heart Association, Inc., mesenchymal stem cells (MSC) have unique properties that make them an intriguing tool for studying brain repair after ischemic stroke. They have the ability to reduce overall inflammation, thereby removing the potentially toxic environment that could be causing NSC death, as well as support NSC survival and function through the secretion of various neurotrophic factors. To date, numerous preclinical studies and clinical trials have demonstrated the efficacy of MSC therapy in stroke preclinical studies and the safety of MSC treatment in clinical trials.
Mesenchymal Stem Cells Market Segmentation
The worldwide market for mesenchymal stem cells containers is split based on products & services, type, workflow type, source of isolation, indication, application, and geography.
Mesenchymal Stem Cells Market By Products & Services
- Products
- Kits, Media, & Reagents
- Cells & Cell Lines
- Others
- Services
According to the mesenchymal stem cells industry analysis, products dominate the market, accounting for a substantial 78% share. This dominance is due to the importance of kits, medium, reagents, cells, and cell lines in MSC research and therapeutic applications. Scientists and physicians use these products to grow, develop, and examine cells. The remaining market share is allocated to services such as cell sourcing, characterization, and cryopreservation. While products now dominate, the services sector is expected to grow as personalized medicine and cell treatments become more prevalent, demanding specialized MSC handling and processing. This expansion will be fueled by the growing demand for high-quality MSCs for clinical trials and pharmaceutical applications.
Mesenchymal Stem Cells Market By Type
- Allogenic MSCs
- Autologous MSCs
Within the services segment, allogenic MSCs now provide the largest income, projected at USD 1.8 billion. Allogenic MSCs produced from a donor have the benefit of being freely available, removing the requirement for patient-specific collection. This makes them a more practical choice for a variety of therapeutic applications. However, autologous MSCs produced from the patient's own body are gaining popularity due to their lower risk of immunological rejection. While autologous MSCs need a more complicated and time-consuming procedure, their individualized character makes them appealing for certain therapy, particularly in regenerative medicine. The mix of allogenic and autologous MSC utilization will most likely be determined by the unique therapeutic reason and the changing cell therapy environment.
Mesenchymal Stem Cells Market By Workflow Type
- Cell Sourcing & Isolation
- Differentiation
- Culture & Cryopreservation
- Characterization
The MSC workflow has several stages, including cell source and separation, differentiation, culture, cryopreservation, and characterization. Currently, culture and cryopreservation are becoming major elements of the workflow. Effective cell culture procedures are critical for preserving MSC viability and increasing cell numbers for research and clinical applications. Cryopreservation, or the freezing and storing of cells, ensures that MSCs are available when needed. As MSC-based therapies progress, optimizing the entire workflow, including quality control and standardization, will become increasingly important to ensuring consistent and beneficial outcomes. This emphasis on workflow efficiency will drive innovation in cell processing technologies, thereby helping to the overall growth of the mesenchymal stem cells market.
Mesenchymal Stem Cells Market By Source of Isolation
- Bone Marrow
- Peripheral Blood
- Cord Blood
- Fallopian Tube
- Lung
- Fetal Liver
- Adipose Tissues
MSCs can be derived from a variety of sources, including bone marrow, peripheral blood, umbilical cord blood, and adipocytes. Although bone marrow was traditionally used, other sources are increasingly prevalent. Adipose tissue offers a less invasive collection method and is widely available. Umbilical cord blood is also a promising supply because it is easy to collect and the cells are quite young. MSC source selection is determined by the specific application and desired cell properties. Ongoing study compares MSCs from various sources to discover the best therapeutic applications.
Mesenchymal Stem Cells Market By Indication
- Bone And Cartilage Repair
- Inflammatory And Immunological Diseases
- Cardiovascular Diseases
- Liver Diseases
- GvHD
- Cancer
- Others
MSCs are being explored for a wide range of therapeutic applications, including bone and cartilage regeneration, inflammatory and immunologic diseases, cardiovascular and liver problems, and even cancer. Bone and cartilage healing, as well as inflammatory and immunological disorders, are important subcategories of the indication category. MSCs' potential to promote tissue regeneration and control the immune system makes them promising options for treating various diseases. As research advances and clinical trials confirm success, the variety of indications for MSC-based therapeutics is projected to broaden, boosting market expansion.
Mesenchymal Stem Cells Market By Application
- Disease Modeling
- Stem Cell Banking
- Drug Development & Discovery
- Toxicology Studies
- Tissue Engineering
- Others
Beyond direct therapeutic applications, MSCs are also used in various research and development activities. These applications include disease modeling, stem cell banking, drug development and discovery, toxicology studies, and tissue engineering. Disease modeling using MSCs allows researchers to study the mechanisms of diseases and develop new treatments. Stem cell banking ensures the availability of MSCs for future use. In drug development, MSCs can be used to assess the safety and efficacy of new drugs. Tissue engineering utilizes MSCs to create functional tissues and organs for transplantation. These diverse applications highlight the versatility of MSCs and their importance in advancing both basic research and translational medicine.
Mesenchymal Stem Cells Market Regional Outlook
North America
- U.S.
- Canada
Europe
- U.K.
- Germany
- France
- Spain
- Rest of Europe
Asia-Pacific
- India
- Japan
- China
- Australia
- South Korea
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Rest of LATAM
The Middle East & Africa
- South Africa
- GCC Countries
- Rest of the Middle East & Africa (ME&A)
Mesenchymal Stem Cells Market Regional Analysis
North America is expected to dominate the global market for mesenchymal stem cells (MSCs). This is due to the rapid growth of technological innovations, high disposable incomes, and well-equipped healthcare centers, all of which will contribute to the growth and development of the MSCs market. Furthermore, high R&D expenditure, the availability of advanced research facilities and skilled professionals, and government initiatives all contribute to the global market's growth.
The Asia-Pacific, on the other hand, is expected to have the fastest growing CAGR in the coming years. Asia is expected to grow rapidly in the global mesenchymal stem cells market over the next five years. China and India are expected to be the Asia-Pacific region's fastest-growing mesenchymal stem cells market. The large pool of patients and rising government funding and support are two key driving forces for the mesenchymal stem cells market in emerging countries. Furthermore, due to increased R&D budgets in Japan, China, and India, the Asia-Pacific market is expected to grow at a rapid CAGR during the mesenchymal stem cell market forecast period.
In addition, Europe is expected to have the second largest market share in the coming years. This is due to the rapid growth of technological innovations, high disposable incomes, and well-equipped healthcare facilities, all of which will contribute to the growth and development of the MSCs market. The implantation of new mesenchymal stem cells in cancer patients is expected to boost the MSC market growth in this region.
Mesenchymal Stem Cells Market Players
Some of the top mesenchymal stem cells companies offered in our report include Lonza, Axol Bioscience Ltd, Cellcolabs, Cyagen, STEMCELL Technologies, Celprogen Corporation, Thermo Fisher Scientific, Inc., PromoCell GmbH, Merck KGaA, Stemedica Cell Technologies, Inc., and Cell Applications, Inc.
Frequently Asked Questions
How big is the mesenchymal stem cells market?
The mesenchymal stem cells market size was valued at USD 3.16 Billion in 2023.
What is the CAGR of the global mesenchymal stem cells market from 2024 to 2032?
The CAGR of mesenchymal stem cells is 12.7% during the analysis period of 2024 to 2032.
Which are the key players in the mesenchymal stem cells market?
The key players operating in the global market are including Lonza, Axol Bioscience Ltd, Cellcolabs, Cyagen, STEMCELL Technologies, Celprogen Corporation, Thermo Fisher Scientific, Inc., PromoCell GmbH, Merck KGaA, Stemedica Cell Technologies, Inc., and Cell Applications, Inc.
Which region dominated the global mesenchymal stem cells market share?
North America held the dominating position in mesenchymal stem cells market during the analysis period of 2024 to 2032.
Which region registered fastest CAGR from 2024 to 2032?
Asia-Pacific region exhibited fastest growing CAGR for market of mesenchymal stem cells during the analysis period of 2024 to 2032.
What are the current trends and dynamics in the global mesenchymal stem cells industry?
The current trends and dynamics in the mesenchymal stem cells market include growing adoption of MSCs for ailments due to aging, and rising prevalence chronic disorders.
Which indication held the maximum share in 2023?
The bone and cartilage healing indication held the notable share of the mesenchymal stem cells industry.?



