Medical Affairs Outsourcing Market Size - Global Industry, Share, Analysis, Trends and Forecast 2024 - 2032
Published :
Report ID:
Pages :
Format :
Medical Affairs Outsourcing Market Size - Global Industry, Share, Analysis, Trends and Forecast 2024 - 2032
Report Coverage
- Industry Dynamics
- Market Size and Forecast Data
- Segment Analysis
- Competitive Landscape
- Regional Analysis with a Niche Focus on Country-Level Data
- High Level Analysis - Porter's, PESTEL, Value Chain, etc.
- Company Profiles of Key Players
- Option to Customize the Report As Per Your Specific Need
Request Sample Report
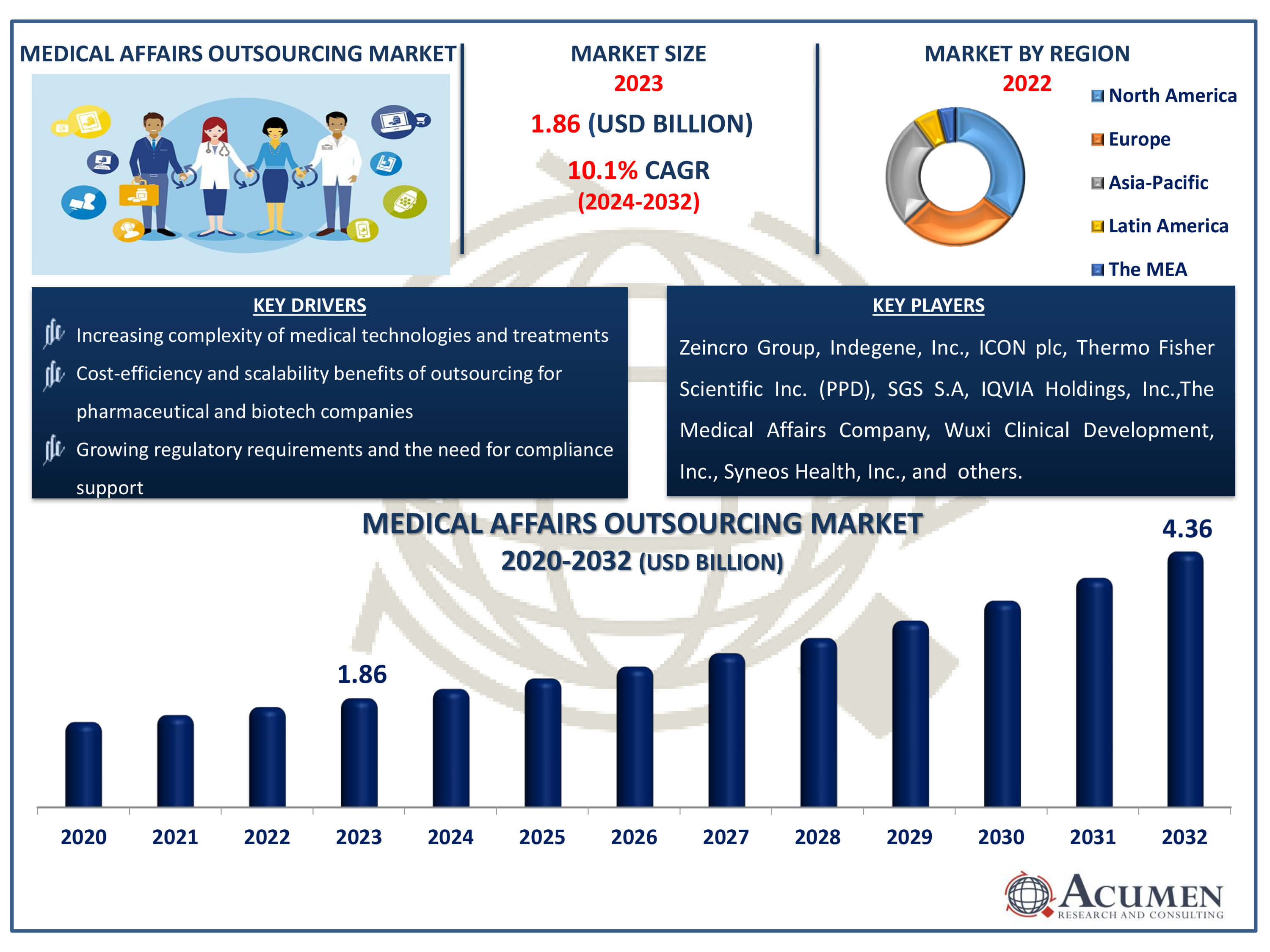
The Medical Affairs Outsourcing Market Size accounted for USD 1.86 Billion in 2023 and is estimated to achieve a market size of USD 4.36 Billion by 2032 growing at a CAGR of 10.1% from 2024 to 2032.
Medical Affairs Outsourcing Market Highlights
- Global medical affairs outsourcing market revenue is poised to garner USD 4.36 billion by 2032 with a CAGR of 10.1% from 2024 to 2032
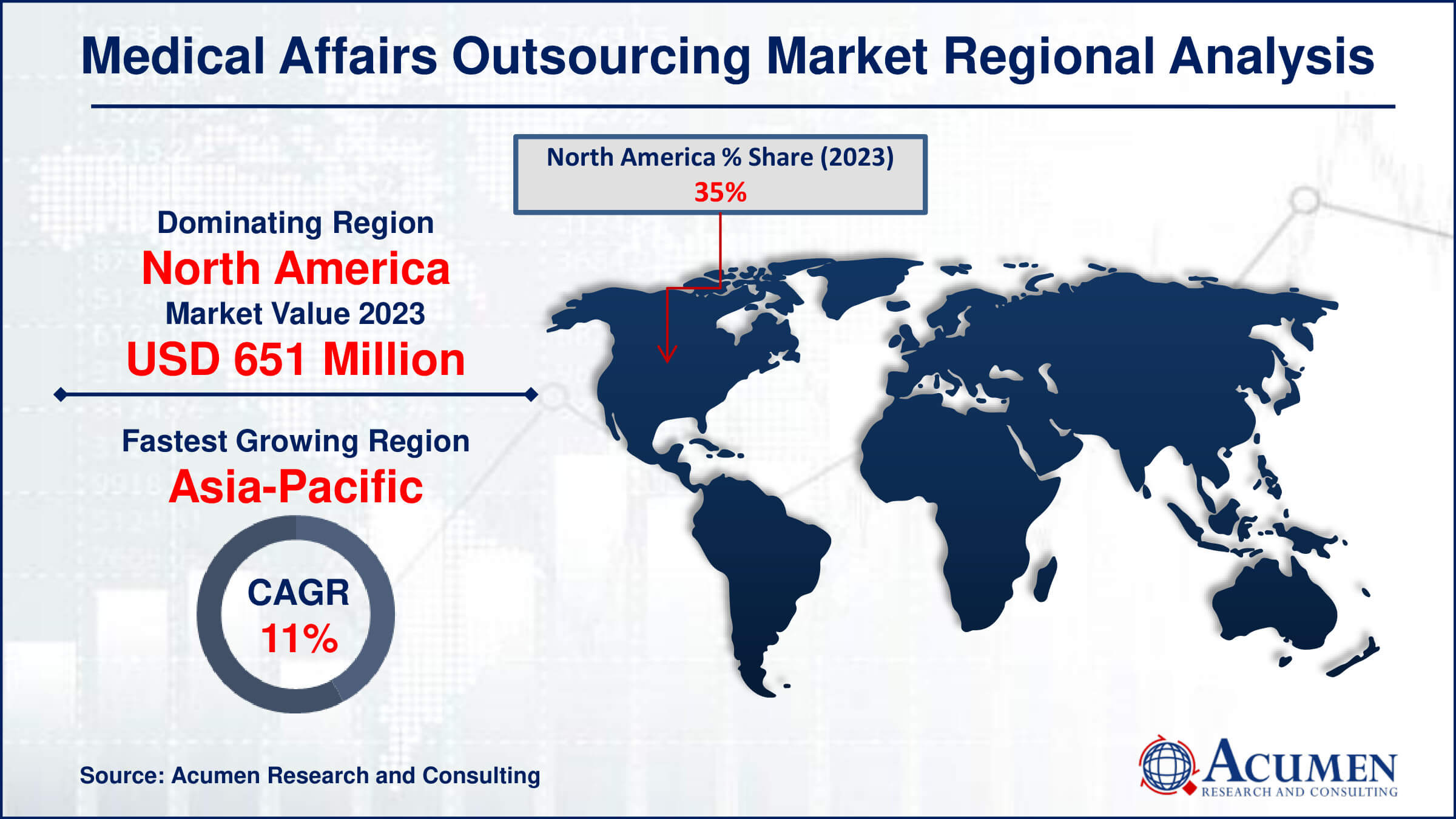
- North America medical affairs outsourcing market value occupied around USD 651 million in 2023
- Asia-Pacific medical affairs outsourcing market growth will record a CAGR of more than 11% from 2024 to 2032
- Among services, the medical writing & publishing sub-segment generated 36% of market share in 2023
- Based on industry, the pharmaceutical sub-segment generated 52% of medical affairs outsourcing market share in 2023
- Increased demand for real-world evidence (RWE) and data analytics is the medical affairs outsourcing market trend that fuels the industry demand
Medical affairs outsourcing entails handing over medical and scientific functions to external specialized service providers. This model allows pharmaceutical and biotechnology businesses to use expertise in areas such as medical writing, regulatory relations, and clinical trial support without having to maintain large in-house teams. Key applications include medical communications, publication planning, compliance management, and health economics and outcomes research (HEOR). Outsourcing increases operational efficiency, guarantees regulatory compliance, and provides access to a worldwide labor pool. It is especially advantageous for small to mid-sized businesses seeking cost-effective solutions and scalability. The medical affairs function is becoming increasingly significant in today's pharmaceutical, biotechnology, medical device, and diagnostic enterprises. This growing position is primarily due to active physicians desire to be and remain clinically aware and educated about new treatment concepts and breakthroughs, keeping them on the cutting edge.
Global Medical Affairs Outsourcing Market Dynamics
Market Drivers
- Increasing complexity of medical technologies and treatments necessitating specialized expertise
- Cost-efficiency and scalability benefits of outsourcing for pharmaceutical and biotech companies
- Growing regulatory requirements and the need for compliance support
Market Restraints
- Concerns over data security and confidentiality in outsourced operations
- Potential loss of control over outsourced functions and quality issues
- High dependency on third-party vendors may lead to disruptions if they underperform
Market Opportunities
- Expansion of personalized medicine and precision therapies requiring specialized medical affairs support
- Technological advancements enabling more efficient and effective outsourcing solutions
- Emerging markets and rising healthcare expenditures creating new demands for outsourced medical affairs services
Medical Affairs Outsourcing Market Report Coverage
| Market | Medical Affairs Outsourcing Market |
| Medical Affairs Outsourcing Market Size 2023 | USD 1.86 Billion |
| Medical Affairs Outsourcing Market Forecast 2032 |
USD 4.36 Billion |
| Medical Affairs Outsourcing Market CAGR During 2024 - 2032 | 10.1% |
| Medical Affairs Outsourcing Market Analysis Period | 2020 - 2032 |
| Medical Affairs Outsourcing Market Base Year |
2023 |
| Medical Affairs Outsourcing Market Forecast Data | 2024 - 2032 |
| Segments Covered | By Product, By Industry, And By Geography |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
| Key Companies Profiled | Indegene, Inc., ICON plc, SGS S.A, IQVIA Holdings, Inc., Wuxi Clinical Development, Inc., The Medical Affairs Company, Zeincro Group, Syneos Health, Inc., Ashfield Healthcare Communications, and Thermo Fisher Scientific Inc. (PPD). |
| Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Covid-19 Analysis, Regulation Analysis |
Medical Affairs Outsourcing Market Insights
Outsourcing in the pharmaceutical and biotech industries offers substantial cost-efficiency and scalability benefits, fueling the growth of the medical affairs outsourcing market. By outsourcing medical affairs, companies can reduce operational costs, access specialized expertise, and adapt swiftly to changing market demands without the need for extensive internal investments. This approach allows for the reallocation of resources towards core competencies such as research and development. Additionally, outsourcing provides flexibility in managing workloads, enabling companies to scale up or down based on project requirements, thereby enhancing overall efficiency and competitiveness in the industry.
However, the growth of the medical affairs outsourcing market is hindered by potential loss of control over outsourced functions, which can lead to inconsistencies in service quality. Companies may struggle to maintain the same standards and regulatory compliance when relying on external providers. This lack of direct oversight can result in errors or miscommunications that impact the overall effectiveness of medical affairs activities. Additionally, the potential for confidential data breaches raises concerns about the security of sensitive information. These challenges make firms hesitant to fully utilize outsourcing, impeding market expansion.
Emerging markets are experiencing significant economic growth, leading to increased healthcare expenditures as populations demand better medical services. This surge in spending is creating opportunities for outsourced medical affairs services, which can provide specialized expertise and cost-effective solutions. Pharmaceutical and biotechnology companies are leveraging these services to navigate complex regulatory environments and improve market access. For instance, PRA Health Sciences Inc was acquired by ICON plc in February 2021 for about USD 12 billion in cash and equity. This acquisition has strengthened the company's medical affairs service offering. Outsourced providers offer comprehensive support in clinical trial management, medical writing, and compliance, enabling firms to focus on core activities. Consequently, the rise in healthcare investments in these markets is driving the expansion of the medical affairs outsourcing industry.
Medical Affairs Outsourcing Market Segmentation
The worldwide market for medical affairs outsourcing is split based on product, Industry, and geography.
Medical Affairs Outsourcing Services
- Medical Writing & Publishing
- Medical Monitoring
- Medical Science Liaisons (MSLs)
- Medical Information
- Others
According to medical affairs outsourcing industry analysis, the medical writing and publishing segment dominates market. This is largely due to the increasing complexity of medical documentation and the growing demand for regulatory compliance and evidence-based communication in the healthcare industry. Companies are outsourcing these tasks to specialized firms to ensure high-quality, accurate, and compliant medical content. The rise of digital health and the expansion of scientific research have further fueled this trend, necessitating expert handling of large volumes of medical information. Consequently, the segment is expected to continue its growth trajectory as the need for professional medical writing and publishing services remains critical.
Medical Affairs Outsourcing Industries
- Pharmaceutical
- Biopharmaceutical
- Medical Devices
- Therapeutic Medical Devices
- Diagnostic Medical Devices
The medical affairs outsourcing industry, the pharmaceuticals industry dominates medical affairs outsourcing market, largely due to the increasing complexity of drug development and regulatory requirements. Pharmaceutical companies are seeking to optimize costs and improve efficiency, leading to a higher demand for specialized external expertise in medical affairs. Outsourcing enables these companies to access advanced technologies and skilled professionals without the overhead of maintaining large in-house teams. This trend is bolstered by the need for enhanced compliance and the growing importance post-market surveillance. Consequently, the pharmaceuticals sector remains at the forefront of outsourcing to meet its evolving needs.
Medical Affairs Outsourcing Market Regional Outlook
North America
- U.S.
- Canada
Europe
- U.K.
- Germany
- France
- Spain
- Rest of Europe
Asia-Pacific
- India
- Japan
- China
- Australia
- South Korea
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Rest of Latin America
The Middle East & Africa
- South Africa
- GCC Countries
- Rest of the Middle East & Africa (ME&A)
Medical Affairs Outsourcing Market Regional Analysis
In terms of medical affairs outsourcing market analysis, North America accounted for the largest market share in the global medical affairs outsourcing market in 2023, and the region is likely to maintain its dominance during the forecast period. Some important reasons contributing to this expansion include the existence of global pharmaceutical and life sciences corporations, strong regulators such as the FDA, and therefore the availability of a skilled pool can contribute to overall growth. Moreover, growing investment in research and development activities which lead to increase demand of medical affairs outsourcing industry in North American region. For instance, the National Institute of Health (NIH) invests considerably in clinical research, with USD 17,610 Billion in 2020, increasing to USD 17,681 Billion in 2021 and USD 18,405 Billion by the end of 2022.
Asia-Pacific is fastest-growing region in medical affairs outsourcing industry. The growing number of patent expirations, together with increased R&D expenditure, is the most important drivers impacting the global regulatory affairs outsourcing industry. The region’s growing pharmaceutical industry and collaborations of robust manufacturers in research and development further contributes to market growth. For instance, according to India Brand Equity Foundation, India boasts the most USFDA-compliant pharmaceutical plants outside of the United States, as well as over 2,000 WHO-GMP accredited facilities, supporting demand from 150+ nations globally, with over 10,500 production facilities. Furthermore, with the investments in healthcare infrastructure and government initiatives boosts demand for medical affairs outsourcing in Asian region.
Medical Affairs Outsourcing Market Players
Some of the top medical affairs outsourcing companies offered in our report includes Indegene, Inc., ICON plc, SGS S.A, IQVIA Holdings, Inc., Wuxi Clinical Development, Inc., The Medical Affairs Company, Zeincro Group, Syneos Health, Inc., Ashfield Healthcare Communications, and Thermo Fisher Scientific Inc. (PPD).
Frequently Asked Questions
How big is the medical affair outsourcing market?
The medical affairs outsourcing market size was valued at USD 1.86 billion in 2023.
What is the CAGR of the global medical affairs outsourcing market from 2024 to 2032?
The CAGR of medical affairs outsourcing is 10.1% during the analysis period of 2024 to 2032.
Which are the key players in the medical affairs outsourcing market?
The key players operating in the global market are including Indegene, Inc., ICON plc, SGS S.A, IQVIA Holdings, Inc., Wuxi Clinical Development, Inc., The Medical Affairs Company, Zeincro Group, Syneos Health, Inc., Ashfield Healthcare Communications, and Thermo Fisher Scientific Inc. (PPD).
Which region dominated the global medical affairs outsourcing market share?
North America held the dominating position in medical affairs outsourcing industry during the analysis period of 2024 to 2032.
Which region registered fastest CAGR from 2024 to 2032?
Asia-Pacific region exhibited fastest growing CAGR for market of medical affairs outsourcing during the analysis period of 2024 to 2032.
What are the current trends and dynamics in the global medical affairs outsourcing industry?
The current trends and dynamics in the medical affairs outsourcing industry include increasing complexity of medical technologies and treatments necessitating specialized expertise, cost-efficiency and scalability benefits of outsourcing for pharmaceutical and biotech companies, and growing regulatory requirements and the need for compliance support.
Which industry held the maximum share in 2023?
The pharmaceutical industry held the maximum share of the medical affairs outsourcing industry.


