IV Bags Material Market Size - Global Industry, Share, Analysis, Trends and Forecast 2024 - 2032
Published :
Report ID:
Pages :
Format :
IV Bags Material Market Size - Global Industry, Share, Analysis, Trends and Forecast 2024 - 2032
Report Coverage
- Industry Dynamics
- Market Size and Forecast Data
- Segment Analysis
- Competitive Landscape
- Regional Analysis with a Niche Focus on Country-Level Data
- High Level Analysis - Porter's, PESTEL, Value Chain, etc.
- Company Profiles of Key Players
- Option to Customize the Report As Per Your Specific Need
Request Sample Report
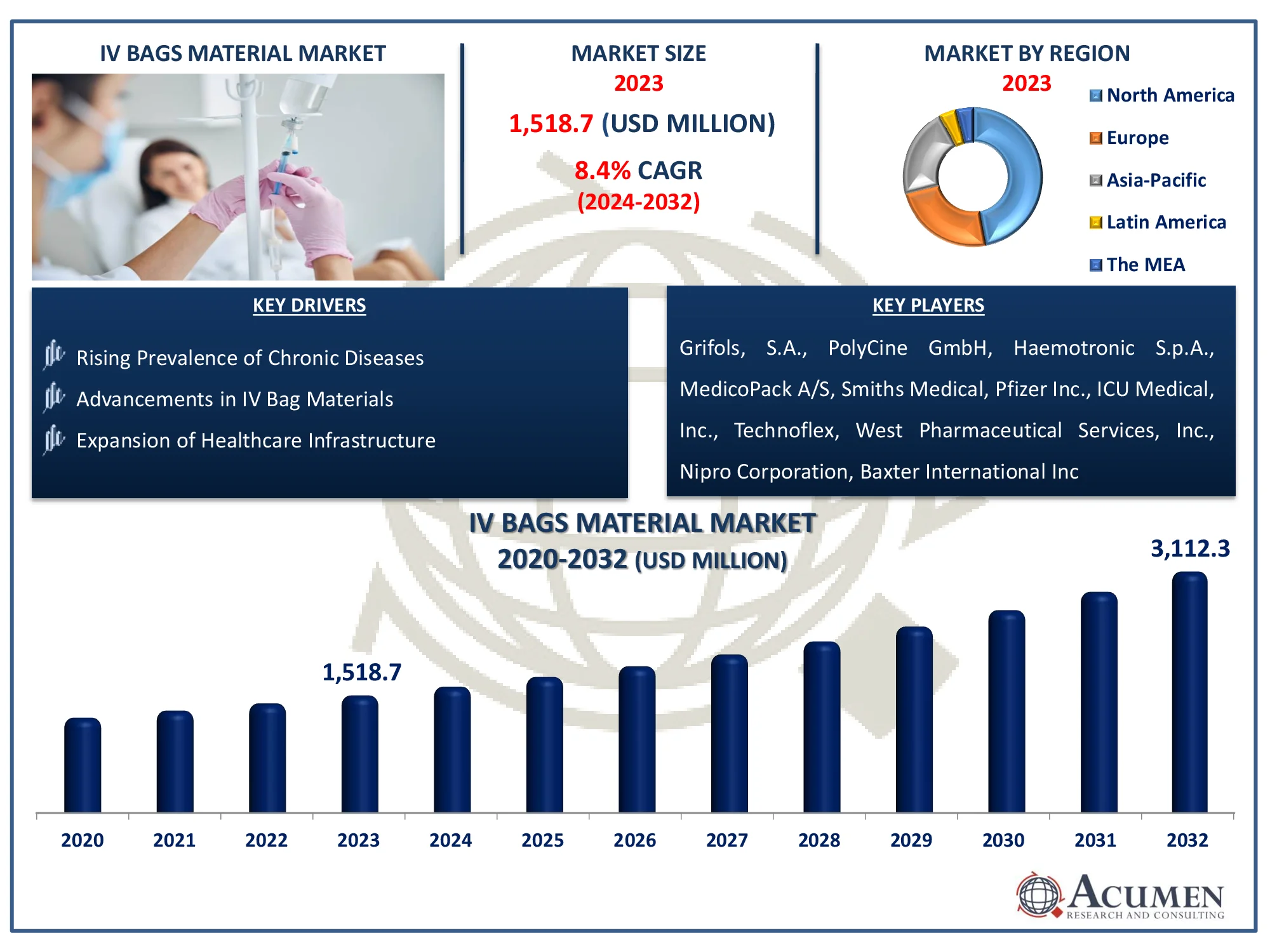
The Global IV Bags Material Market Size accounted for USD 1,518.7 Million in 2023 and is estimated to achieve a market size of USD 3,112.3 Million by 2032 growing at a CAGR of 8.4% from 2024 to 2032.
IV Bags Material Market Highlights
- The global IV bags material market is expected to reach USD 3,112.3 million by 2032, growing at a CAGR of 8.4% from 2024 to 2032
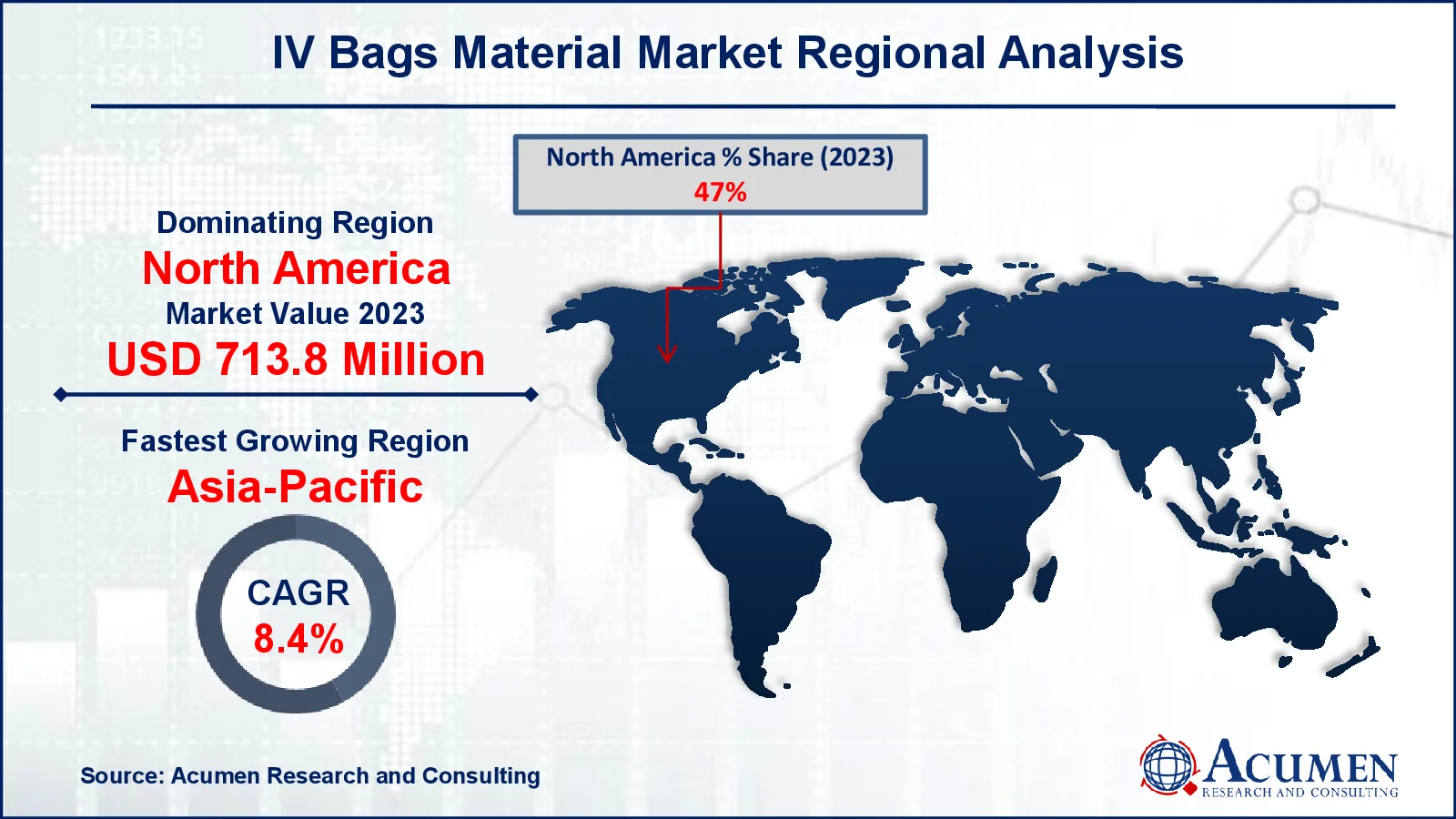
- In 2023, North America's IV bags material market was valued at approximately USD 713.8 million
- The Asia-Pacific region is projected to achieve a CAGR exceeding 9.2% from 2024 to 2032
- The single chamber sub-segment accounted for 65% of the market share in 2023
- Increasing hospitalization and surgical procedures is the IV bags material market trend that fuels the industry demand
IV (intravenous) bags are medical devices that administer fluids, medicines, and nutrients directly into a patient's bloodstream. They are often made of flexible, robust materials that promote patient safety and sterility during medical procedures. The most common IV bag materials are polyvinyl chloride (PVC), polyolefin (such as polyethylene and polypropylene), and ethylene vinyl acetate (EVA). PVC is widely utilized due to its flexibility and transparency, although worries about leaking plasticizers such as DEHP have prompted the development of substitutes. Polymer ole bags are becoming increasingly popular because they are free of toxic plasticizers, making them safer for patients, particularly neonates and that requiring long-term infusion therapy. EVA is another non-PVC material noted for its chemical resilience and compatibility with a variety of medications.
Global IV Bags Material Market Dynamics
Market Drivers
- Increasing hospital admissions drive demand for IV therapies and materials
- Shift toward biocompatible, DEHP-free, and non-PVC alternatives enhances market growth
- Growing investments in hospitals and clinics boost IV bag consumption globally
Market Restraints
- PVC-based IV bags contribute to medical waste and regulatory scrutiny worldwide
- Raw material shortages and logistical challenges impact IV bag material availability
- Stringent compliance requirements delay approvals and increase manufacturing costs
Market Opportunities
- Rising demand for sustainable, recyclable, and non-toxic IV bag materials
- Healthcare modernization in developing regions fuels IV bag material demand
- Advanced polymer formulations enhance durability, safety, and drug compatibility
IV Bags Material Market Report Coverage
|
Market |
IV Bags Material Market |
|
IV Bags Material Market Size 2023 |
USD 1,518.7 Million |
|
IV Bags Material Market Forecast 2032 |
USD 3,112.3 Million |
|
IV Bags Material Market CAGR During 2024 - 2032 |
8.4% |
|
IV Bags Material Market Analysis Period |
2020 - 2032 |
|
IV Bags Material Market Base Year |
2023 |
|
IV Bags Material Market Forecast Data |
2024 - 2032 |
|
Segments Covered |
By Material Type, By Chamber Type, By Size, By End-User, and By Geography |
|
Regional Scope |
North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
|
Key Companies Profiled |
Grifols, S.A., PolyCine GmbH, Haemotronic S.p.A., MedicoPack A/S, Smiths Medical, Pfizer Inc., ICU Medical, Inc., Technoflex, West Pharmaceutical Services, Inc., Nipro Corporation, Baxter International Inc., Fresenius Kabi AG, Amcor plc, Kraton Corporation, Sippex (A MEDIPPEX Co.), B. Braun Melsungen AG, Fosmo Med, JW Life Science Corp., and Otsuka Pharmaceutical Co., Ltd. |
|
Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Covid-19 Analysis, Regulation Analysis |
IV Bags Material Market Insights
The rising prevalence of chronic illnesses such as diabetes, cardiovascular disease, and cancer needs ongoing intravenous therapy, raising demand for IV bags. For instance, according to the Centers for Disease Control and Prevention, an estimated 129 million people in the United States have at least one significant chronic condition (such as heart disease, cancer, diabetes, obesity, or hypertension), as defined by the US Department of Health and Human Services. Additionally, CDC states persons under the age of 65 will account for around one out of every five deaths from cardiovascular diseases (CVDs) in 2022. Coronary heart disease is the most prevalent form of heart disease. It killed 371,506 people in 2022. Every 33 seconds, one person dies from cardiovascular disease. The increasing need for IV bags for patients recovering from chronic illnesses drives market expansion for IV bag materials.
The discovery and adoption of non-PVC materials, such as polypropylene and ethylene vinyl acetate, answer concerns about patient safety and environmental effect, resulting in increased acceptability and use. For example, the Health Care Without Harm Organization believes that European politicians must limit the use of PVC and its toxic compounds while also stimulating demand for PVC-free alternatives. The use of PVC-free materials such as silicone, polyolefins, polyethylene, polypropylene, polyethylene, or polyurethane avoids the need for potentially toxic plasticizers or additives, as well as the risks associated with the manufacture, use, and disposal of PVC medical devices. The shift to non-PVC materials is gaining traction, and their application in the medical field increases demand for safer alternatives.
The use of traditional materials in IV bags, such as polyvinyl chloride (PVC), presents environmental concerns due to recycling challenges and the potential release of hazardous compounds during disposal. This may impede market expansion in the forecast year due to environmental concerns. Furthermore, developing market expansion driven by key manufacturers contributes to increased usage of IV bags, which will represent an opportunity in the near future. For example, in March 2021, Fagron Sterile Services (FSS) launched a new platform that includes different presentations of fentaNYL, HYDROmorphone, and midazolam for intravenous (IV) bags. FSS intended to expand its product line with IV Bag drugs such as oxytocin, NORepinephrine, Vancomycin, Ketamine, and PHENYLephrine. Furthermore, investing in research and development to develop biodegradable or easily recyclable IV bag materials can address environmental issues while meeting the growing need for sustainable healthcare solutions.
IV Bags Material Market Segmentation
The worldwide market for IV bags material is split based on material type, chamber type, size, end-user, and geography.
IV Bag Materials Market By Material Type
- Polypropylene (PP)
- Polyethylene (PE)
- Ethylene Vinyl Acetate (EVA)
- Polyvinyl Chloride (PVC)
- Copolyester Ether
- Others
According to the IV bags material industry analysis, polyvinyl chloride (PVC) has long dominated the market due to its flexibility, durability, and cost-effectiveness. However, worries about plasticizer leaking and environmental difficulties have resulted in a gradual transition toward non-PVC alternatives. Materials including polypropylene (PP), polyethylene (PE), and ethylene vinyl acetate (EVA) are gaining popularity due to their safer properties and lower environmental effect. This change is also aided by regulatory pressures and an increased emphasis on patient safety and sustainability.
IV Bag Materials Market By Chamber Type
- Single Chamber
- Multiple Chamber
According to the IV bags material industry analysis, single-chamber bags are becoming more popular because of their ease of use, low cost, and broad use in conventional IV therapy. They are widely utilized for basic fluid administration, electrolyte replenishment, and medicine delivery, making them the preferred option in the majority of healthcare settings. Their simplicity of manufacture and little risk of contamination add to their dominance. Multiple-chamber IV bags are becoming increasingly used for specialty treatments including parenteral nutrition and combination medication therapy, as separate sections prevent premature mixing. Although they have a lesser market share, they are being used in advanced medical care and personalized medicine applications.
IV Bag Materials Market By Size
- 250 ml to 500 ml
- Less than 250 ml
- 500 ml to 1000 ml
- More than 1000 ml
According to the IV bags material industry analysis, 500 ml to 1000 ml sector has a considerable market share, thanks to its broad use in medical applications such as fluid resuscitation and medication delivery. This size range strikes a compromise between volume and manageability, making it an ideal choice in clinical settings. The less than 250 ml and 250 ml to 500 ml segments are commonly used for pediatric care, accurate drug administration, and specific treatments that require smaller fluid quantities. The more than 1000 ml section is used in instances requiring significant fluid delivery, such as major procedures or severe dehydration.
IV Bag Materials Market By End-User
- Hospitals
- Clinics
- Ambulatory Surgical Centers
- Others
According to the IV bags material market foreacst, hospitals dominate because of their large patient population, numerous medical procedures, and ongoing demand for intravenous fluids and drugs. Their sturdy infrastructure and excellent healthcare facilities enable widespread use of IV bags for hydration therapy, pharmaceutical management and blood transfusions. Furthermore, hospitals' capacity to handle complex medical cases maintains ongoing demand. Clinics and ambulatory surgical facilities follow, fueled by the increased demand for outpatient care and minimally invasive surgeries.
IV Bags Material Market Regional Outlook
North America
- U.S.
- Canada
Europe
- U.K.
- Germany
- France
- Spain
- Rest of Europe
Asia-Pacific
- India
- Japan
- China
- Australia
- South Korea
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Rest of LATAM
The Middle East & Africa
- South Africa
- GCC Countries
- Rest of the Middle East & Africa (ME&A)
IV Bags Material Market Regional Analysis
For several reasons, North America leads the IV bag material market due to its advanced healthcare infrastructure, high healthcare spending, and widespread use of intravenous therapy. For instance, as per Centers for Medicare & Medicaid Services, in 2023, US health-care spending increased by 8.4% to $4.9 trillion, or $14,570 per person. Health spending made for 17.6 percent of the nation's GDP. The presence of prominent medical device manufacturers, as well as tight regulatory criteria, ensures product quality and safety. For example, in July 2024, Amneal Pharmaceuticals reported that the US FDA has approved a New Drug Application (NDA) for its new potassium phosphates in 0.9% sodium chloride injection IV ready-to-use bags. The injection provides phosphorus to adults and pediatric patients with hypophosphatemia. The approval of ready-to-use bags is projected to boost the IV bag materials market by raising demand for sophisticated and dependable packaging solutions.
Asia-Pacific is expanding rapidly due to increased healthcare investments, rising population, and improved medical facilities. The rising innovation in IV bags by major pharmaceutical companies increases demand for IV bags. For example, in December 2022, Asahi Kasei Pharma introduced Reclast for intravenous (IV) infusion bags for osteoporosis treatment, which is included on Japan's National Health Insurance (NHI) medicine price standard. Furthermore, the transition to contemporary healthcare practices, as well as government attempts to improve healthcare access, are driving rapid market expansion in this region.
IV Bags Material Market Players
Some of the top IV bags material companies offered in our report include Grifols, S.A., PolyCine GmbH, Haemotronic S.p.A., MedicoPack A/S, Smiths Medical, Pfizer Inc., ICU Medical, Inc., Technoflex, West Pharmaceutical Services, Inc., Nipro Corporation, Baxter International Inc., Fresenius Kabi AG, Amcor plc, Kraton Corporation, Sippex (A MEDIPPEX Co.), B. Braun Melsungen AG, Fosmo Med, JW Life Science Corp., and Otsuka Pharmaceutical Co., Ltd.
Frequently Asked Questions
How big is the IV Bags Material market?
The IV bags material market size was valued at USD 1,518.7 Million in 2023.
What is the CAGR of the global IV Bags Material market from 2024 to 2032?
The CAGR of IV bags material is 8.4% during the analysis period of 2024 to 2032.
Which are the key players in the IV Bags Material market?
The key players operating in the global market are including Grifols, S.A., PolyCine GmbH, Haemotronic S.p.A., MedicoPack A/S, Smiths Medical, Pfizer Inc., ICU Medical, Inc., Technoflex, West Pharmaceutical Services, Inc., Nipro Corporation, Baxter International Inc., Fresenius Kabi AG, Amcor plc, Kraton Corporation, Sippex (A MEDIPPEX Co.), B. Braun Melsungen AG, Fosmo Med, JW Life Science Corp., and Otsuka Pharmaceutical Co., Ltd.
Which region dominated the global IV Bags Material market share?
North America held the dominating position in IV bags material industry during the analysis period of 2024 to 2032.
Which region registered fastest growing CAGR from 2024 to 2032?
Asia-Pacific region exhibited fastest growing CAGR for market of IV bags material during the analysis period of 2024 to 2032.
What are the current trends and dynamics in the global IV Bags Material industry?
The current trends and dynamics in the IV bags material industry include increasing hospital admissions drive demand for IV therapies and materials, shift toward biocompatible, DEHP-free, and non-PVC alternatives enhances market growth, and growing investments in hospitals and clinics boost IV bag consumption globally.
Which chamber type held the maximum share in 2023?
The single chamber held the maximum share of the IV bags material industry.?



