In Vitro Diagnostics Market | Acumen Research and Consulting
In-Vitro Diagnostics Market Size - Global Industry, Share, Analysis, Trends and Forecast 2022 - 2030
Published :
Report ID:
Pages :
Format :
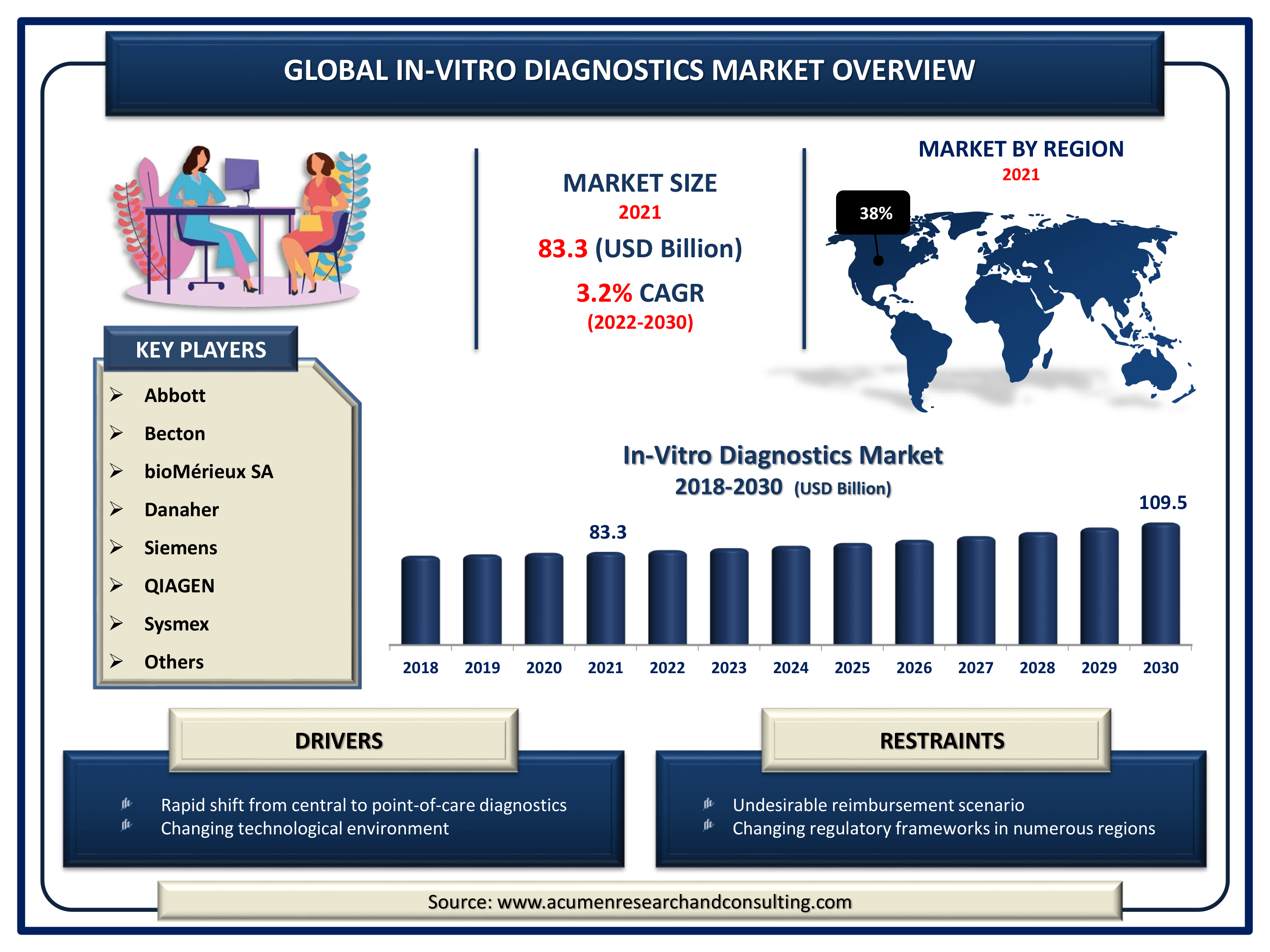
The Global In-Vitro Diagnostics Market Size accounted for USD 83.3 Billion in 2021 and is estimated to achieve a market size of USD 109.5 Billion by 2030 growing at a CAGR of 3.2% from 2022 to 2030. The growth of the in-vitro diagnostics market value can be attributed to the increased incidence of chronic and infectious diseases worldwide. The development of computerized IVD solutions for laboratories & hospitals in order to offer fast, precise, and error-free diagnoses is likely to drive the in-vitro diagnostics market growth. Furthermore, technical progress, such as quick testing kits for the IVD industry, has accelerated in recent years and is projected to continue in the coming years.
In-Vitro Diagnostics Market Report Key Highlights
- Global in-vitro diagnostics market revenue intended to expand USD 109.5 billion by 2030 with a CAGR of 3.2% from 2022 to 2030
- North America in-vitro diagnostics market accounted for over 38% regional shares in 2021
- Based on product, reagent segment accounted for more than half of total market share in 2021
- Among applications, infectious disease segment held the 60% market share in 2021
- In vitro diagnostics tests influence around 70% of clinical choices, according to the British In-vitro Diagnostic Association
- Rising need for enhanced molecular diagnostics testing will drive the in-vitro diagnostics market statistics
In-vitro diagnostics (IVDs) are tests performed on samples involving blood or tissues that are taken from the human body. In-vitro diagnostics detect diseases or other conditions and can be used to monitor a person's overall health to treat, cure, and prevent diseases. Furthermore, IVDs are devices defined under section 201(h) of the Federal Food, Drug, and Cosmetic Act and are also considered biological products subject to section 351 of the Public Health Services Act. Like other medical devices, IVDs are subjected to premarket and post-market controls. Moreover, IVDs are subjected to categorization under the "Clinical Laboratory Improvement Amendments (CLIA '88) of 1988.
COVID-19 accelerates demand for IVD
With high technological and regulatory barriers, in-vitro diagnostics (IVD) has grabbed significant attention coupled with the relatively high-margin industry with molecular diagnostics being one of the fastest growing segments. With the spread of the pandemic, the demand for in-vitro diagnostics has increased. Additionally, point-of-care (POC) testing has risen during the COVID-19 pandemic in response to the demand for faster on-site screening. According to the Rockefeller Foundation, estimates show that around 70 million POC tests were conducted in the US with a strong peak reaching 200 million a month in the forecast period. The growing number of patients in the pandemic gives rise to the development of new diagnostic technologies that involve next-generation sequencing (NGS) and CRISPR. NGS has benefited from regulatory support. Authorities in China and US have approved some NGS-based COVID-19 diagnostics for emergency use. Others are in pipeline with throughput as high as 100,000 samples per run.
Global In-Vitro Diagnostics Market Dynamics
Market Drivers
- The rising elderly population and consequent increase in chronic and communicable diseases incidence
- Rising awareness and endeavors to improve access to healthcare in emerging nations
- The rapid shift from central to point-of-care diagnostics
- Changing technological environment
Market Restraints
- Undesirable reimbursement scenario
- Changing regulatory frameworks in numerous regions
Market Opportunities
- Designing disease-specific biomarkers and assays
- The increasing importance of diagnostics and therapeutics
In-Vitro Diagnostics Market Report Coverage
| Market | In-Vitro Diagnostics Market |
| In-Vitro Diagnostics Market Size 2021 | USD 83.3 Billion |
| In-Vitro Diagnostics Market Forecast 2030 | USD 109.5 Billion |
| In-Vitro Diagnostics Market CAGR During 2022 - 2030 | 3.2% |
| In-Vitro Diagnostics Market Analysis Period | 2018 - 2030 |
| In-Vitro Diagnostics Market Base Year | 2021 |
| In-Vitro Diagnostics Market Forecast Data | 2022 - 2030 |
| Segments Covered | By Product, By Technology, By Application, By End-Use, And By Geography |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, and Middle East & Africa |
| Key Companies Profiled | Abbott, Becton, Dickinson and Company, bioMérieux SA, Bio-Rad Laboratories, Inc., Danaher (Beckman Coulter, Inc.), F. Hoffmann-La Roche AG, Siemens, QIAGEN, Sysmex, and Thermo Fisher Scientific. |
| Report Coverage |
Market Trends, Drivers, Restraints, Competitive Analysis, Player Profiling, Regulation Analysis |
Future Of In-Vitro Diagnostics
As per the American Association for Clinical Chemistry, non-invasive prenatal testing (NIPT), next-generation sequencing (NGS), liquid biopsy, and circulating tumor cells (CTC) tests are the five strong pillars of the in-vitro diagnostics (IVD) industry. According to the statistics revealed by Kalorama Information, publisher of the Worldwide Market for In Vitro Diagnostic Tests, estimates that over the next few years, sales using NGS will witness tremendous growth from US$ 250 Mn to US$ 800 Mn, with the number of CTC tests rising from US$ 100 Mn to US$ 300 Mn. Furthermore, a molecular diagnostic is a fast-growing segment in the global market coupled with some of the larger IVD companies involved in this area. Expansion of the lab-developed tests (LDTs) and more commercialization of reagents and test kits will grab significant turnover for the global market.
Development Of Automated In-Vitro Diagnostic Will Gain 100% Results In The Forecast Period
A diagnostic test for COVID-19 designed for use in a diagnostic system can process up to 1,000 tests in 24 hours. However, laboratory-based tests typically take at least 24 hours to reveal the results once the samples reach the laboratory. Rapid, high-throughput tests are critical to offering quick results for the global population to aid worldwide public health response. Therefore, the molecular diagnostic test from Hologic, Inc. became the first COVID-19 product selected for development through ASPR's Biomedical Advanced Research and Development Authority. Additionally, Biomedical Advanced Research and Development Authority (BARDA) contributed US$ 699,000 to accelerate Hologic's development of a test that detects the genetic material of SARS-CoV-2. For product licensure and to reduce the spread of COVID-19, federal agencies are focusing on identifying products and technologies that will progress beyond non-clinical studies and establish domestic large-scale commercial Good Manufacturing Practices (cGMP) manufacturing capability and utilization of a platform to manufacture a product already approved by the FDA.
The Rising Number Of In-Vitro Diagnostic Products Launched By Prominent Players Fuels The Global IVD Market
The manufacturers have expanded their manufacturing facilities across various regions involving Europe, North America, Latin America, and RoW. COVID-19 had a strong influence on the business. In September 2020, F. Hoffmann-La Roche Ltd announced the launching of a new product namely the SARS-CoV-2 Rapid Antigen Test in countries with CE mark that enhances fast triage decisions at point-of-care. Additionally, in November 2020, Sysmex Corporation, announced receiving in vitro diagnostic approval for manufacturing and marketing of a SARS coronavirus antigen kit HISCLTM SARS-CoV-2 Ag Reagent, which, in conjunction with its fully automated immunoassay systems HISCLTM-5000 / HISCLTM-800. The company launched the product in the commercial market on November 18, 2020.
In-Vitro Diagnostics Market Segmentation
The worldwide in-vitro diagnostics market segmentation is based on the product, technology, application, end-Use, and geography.
In-Vitro Diagnostics Market By Product
- Instruments
- Reagents
- Services
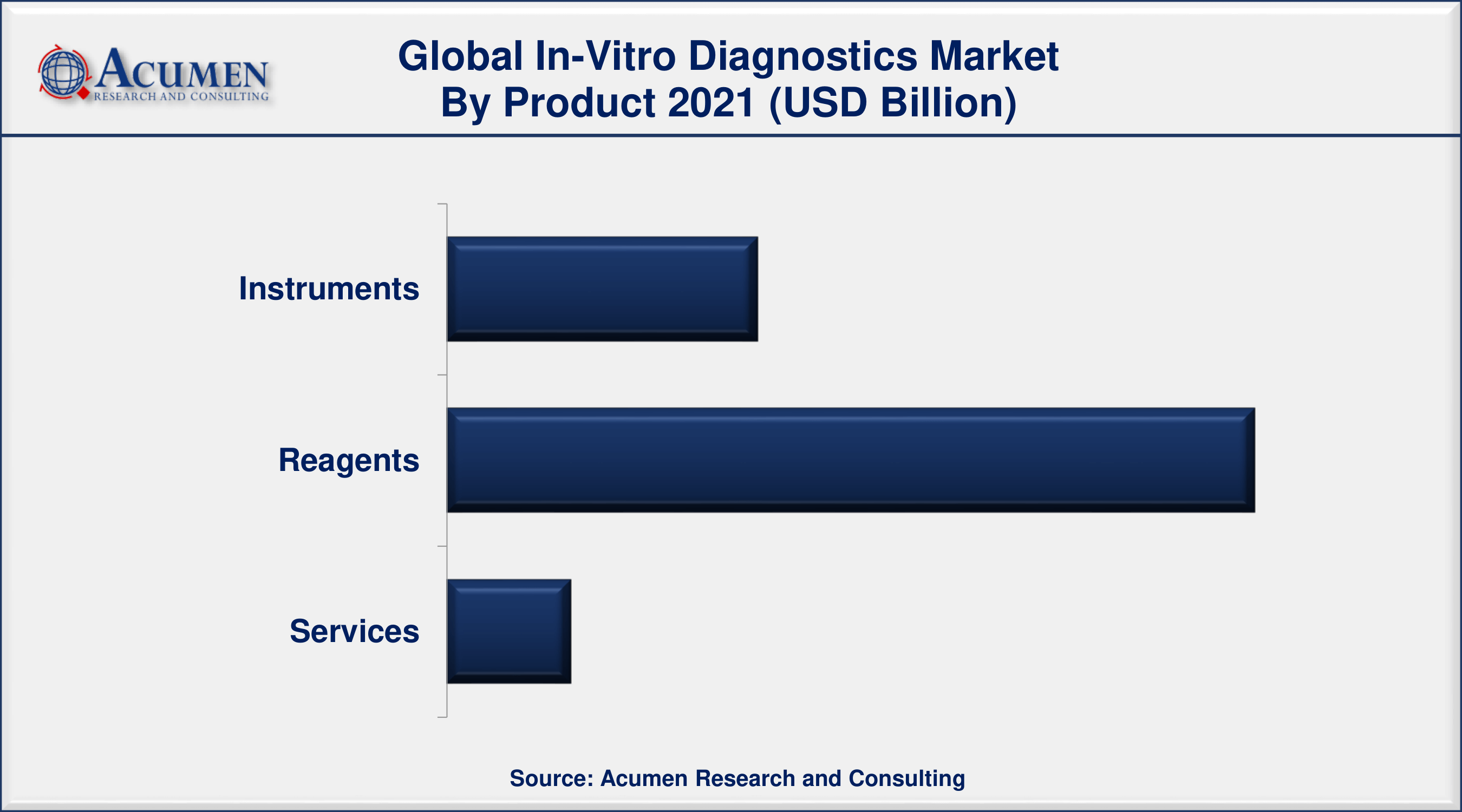
Based on product, the reagent segment dominated the overall IVD market and will continue its trend in the forthcoming years by recording a significant revenue share for the in-vitro diagnostics market. Furthermore, by application segment, the infectious diseases segment holds the dominating share and will account maximum market share contributing to the growth of the overall IVD market. Based on end-user, the hospital segment is dominating the market and will continue its trend in the coming years. Such prominent factors contribute to the fullest to the ultimate growth of the in-vitro diagnostic market globally.
In-Vitro Diagnostics Market By Technology
- Immunoassay
- Hematology
- Clinical Chemistry
- Molecular Diagnostics
- Coagulation
- Microbiology
- Others
According to the in-vitro diagnostics industry analysis, the molecular diagnostics segment held the largest market share in 2021. This expansion is due to the introduction of new items and the constant advancement of technology. Although PCR often requires sophisticated instruments, research initiatives in this field have resulted in advances including plasmofluidic chips in the area of a postage stamp.
In-Vitro Diagnostics Market By Application
- Infectious Disease
- Diabetes
- Oncology
- Cardiology
- Nephrology
- Autoimmune Disease
- Drug testing
- Others
In terms of application, the infectious disease segment is predicted to increase rapidly and account for more than half of the market by 2021. Microorganisms that induce infectious disorders can be detected using IVDs. HIV/AIDS, TB, hepatitis, as well as pneumonia, are the most frequent life-threatening illnesses. Furthermore, prominent market participants are collaborating to promote to patients and healthcare providers the availability of high-quality, novel laboratory services.
In-Vitro Diagnostics Market By End-Use
- Hospitals
- Laboratories
- Home Care
- Others
According to the in-vitro diagnostics market forecast, the laboratory segment is projected to lead the market in the coming years. Increased awareness of personalized medicine, increased need for cheap services, and technological improvements are some of the key reasons projected to drive laboratory segment growth. Due to the huge volume of testing performed in laboratories, this is among the market's most lucrative segments.
In-Vitro Diagnostics Market Regional Outlook
North America
- U.S.
- Canada
Europe
- U.K.
- Germany
- France
- Spain
- Rest of Europe
Latin America
- Mexico
- Brazil
- Rest of Latin America
Asia-Pacific
- India
- Japan
- China
- Australia
- South Korea
- Rest of Asia-Pacific
The Middle East & Africa (MEA)
- Gulf Cooperation Council (GCC)
- South Africa
- Rest of the Middle East & Africa
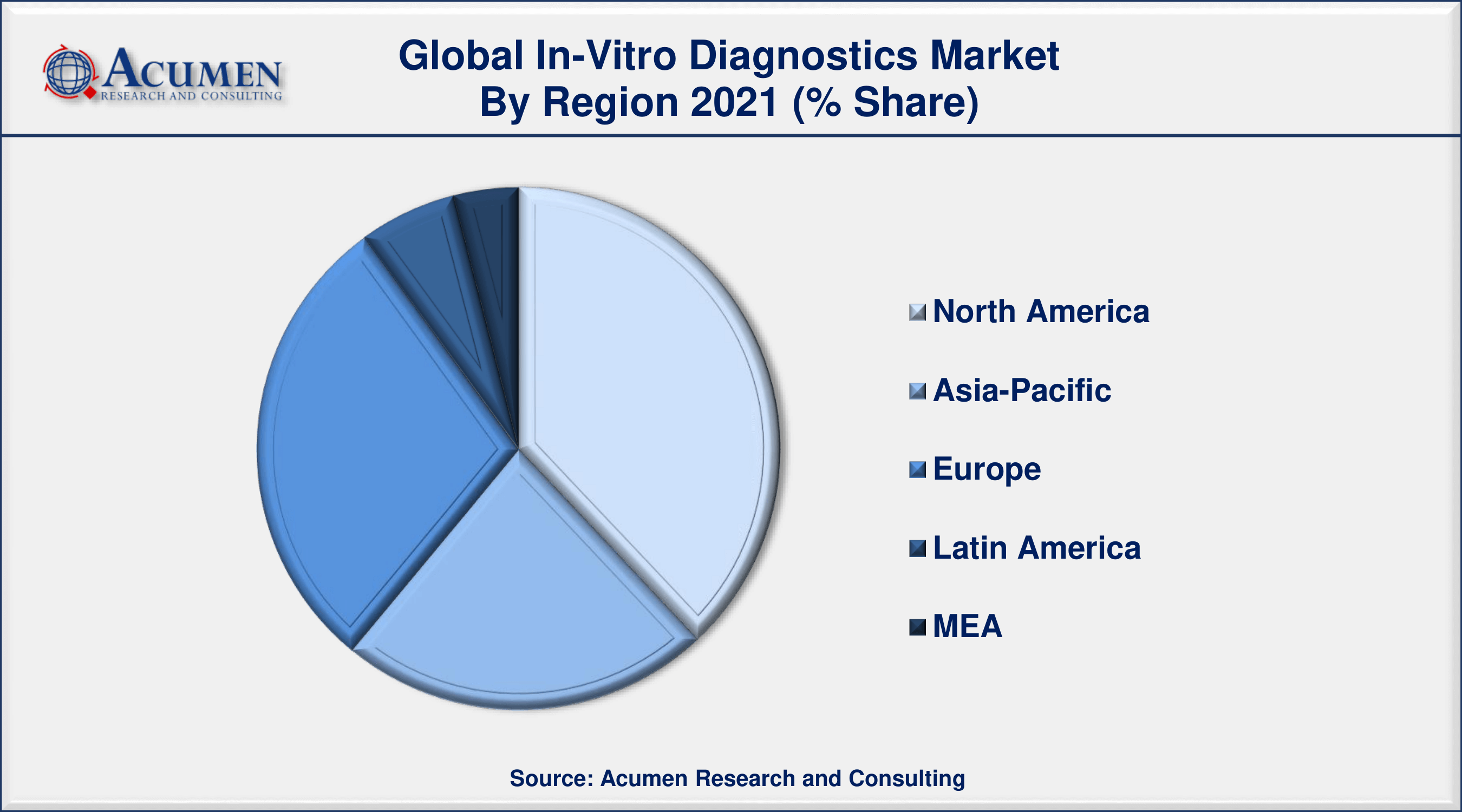
Based on regions, North America holds the dominating market share in the in-vitro diagnostics market. The presence of prominent players involved in the manufacturing of in-vitro diagnostics, high investments, and increasing focus on the POC and molecular diagnostics coupled with the surge in R&D are the key factors that contribute to the fullest the growth of North America regional market for in-vitro diagnostics. On the other hand, Asia-Pacific will record a CAGR of an all-time high during the forecast period for the in-vitro diagnostic market. Improvement in the regulatory scenario and surging affordability rates are the prominent factors for the growth of the in-vitro diagnostics market in the Asia-Pacific region.
In-Vitro Diagnostics Market Players
Some of the top in-vitro diagnostics companies offered in the professional report include Abbott, Becton, Dickinson and Company, bioMérieux SA, Bio-Rad Laboratories, Inc., Danaher (Beckman Coulter, Inc.), F. Hoffmann-La Roche AG, Siemens, QIAGEN, Sysmex, and Thermo Fisher Scientific.
Frequently Asked Questions
What is the size of global in-vitro diagnostics market in 2021?
The estimated value of global in-vitro diagnostics market in 2021 was accounted to be USD 83.3 Billion.
What is the CAGR of global in-vitro diagnostics market during forecast period of 2022 to 2030?
The projected CAGR in-vitro diagnostics market during the analysis period of 2022 to 2030 is 3.2%.
Which are the key players operating in the market?
The prominent players of the global in-vitro diagnostics market are Abbott, Becton, Dickinson and Company, bioMérieux SA, Bio-Rad Laboratories, Inc., Danaher (Beckman Coulter, Inc.), F. Hoffmann-La Roche AG, Siemens, QIAGEN, Sysmex, and Thermo Fisher Scientific.
Which region held the dominating position in the global in-vitro diagnostics market?
North America held the dominating in-vitro diagnostics during the analysis period of 2022 to 2030.
Which region registered the fastest growing CAGR for the forecast period of 2022 to 2030?
Asia-Pacific region exhibited fastest growing CAGR for in-vitro diagnostics during the analysis period of 2022 to 2030.
What are the current trends and dynamics in the global in-vitro diagnostics market?
Rising elderly population and consequent increase in chronic and communicable diseases incidence drives the growth of global in-vitro diagnostics market.
By application segment, which sub-segment held the maximum share?
Based on application, infectious disease segment is expected to hold the maximum share in-vitro diagnostics market.



