Medical Devices Vigilance Market Size to Reach USD 176.1 Billion by 2032 growing at 9.7% CAGR - Exclusive Report by Acumen Research and Consulting
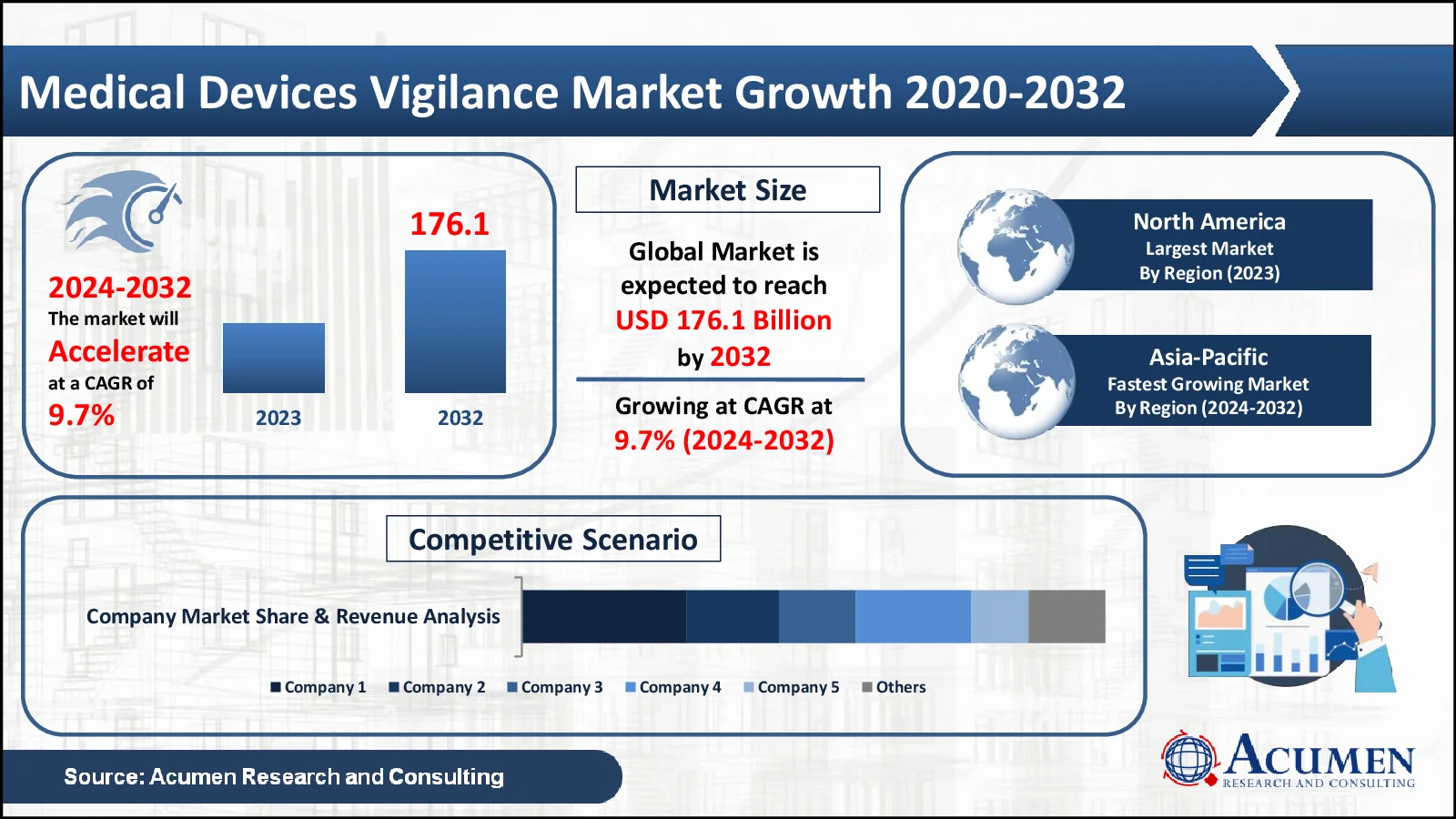
The Medical Devices Vigilance market, valued at USD 76.9 Billion in 2023, is projected to surpass USD 176.1 Billion by 2032, indicating a robust CAGR of 9.7%
The market focuses on the monitoring, reporting, and resolution of safety hazards associated with medical devices after they have been marketed. With increased regulatory scrutiny and the growing relevance of patient safety, producers must build comprehensive post-market surveillance systems. These systems aid in the detection of adverse events, device failures, and other potential dangers to patient safety. The expanding usage of complicated technologies, combined with advances in digital health, has increased the demand for effective vigilance mechanisms. This market is predicted to expand as regulatory authorities tighten restrictions and demand for real-time monitoring systems rises worldwide.
However, varying and complex regulatory regulations across different countries impede market expansion. Furthermore, the increasing need for real-time monitoring and remote surveillance solutions has fueled growth in the medical device vigilance market. Manufacturers encounter difficulty in maintaining compliance with several standards, which slows growth attempts. Despite these challenges, the increased emphasis on continuous monitoring and improved surveillance technologies presents tremendous opportunities. As healthcare institutions increasingly use digital tools, the market for vigilance solutions evolves and grows.
Medical Devices Vigilance Market Statistics
- The global medical devices vigilance market generated USD 76.9 billion in 2023 and is projected to grow at a robust CAGR of over 9.7% from 2024 to 2032
- North America led the market in 2023, contributing USD 26.1 billion in revenue
- The Asia-Pacific region is expected to experience significant growth, with a projected CAGR of 10.5%
- The on-demand delivery mode held the largest market share, accounting for 81% in 2023
- The diagnostic sub-segment dominated the market, capturing 36% of the share in 2023
- A discernible trend in the medical devices vigilance market is greater focus on patient-centric design is shaping device development and reporting
Access Table Of Content: https://www.acumenresearchandconsulting.com/table-of-content/medical-devices-vigilance-market
Medical Devices Vigilance Market Dynamics
Growing Emphasis on Patient Safety and Post-Market Surveillance Fuels the Medical Devices Vigilance Market Value
The growing emphasis on patient safety and the need for improved post-market surveillance are driving the medical device vigilance market. For instance, in September 2023, the US President's Council of Advisors on Science and Technology (PCAST) issued a report titled "A Transformational Effort on Patient Safety" that included suggestions to further the country's commitment to supporting comprehensive safety solutions for patients and the health care workforce.. Regulatory bodies throughout the world have tightened safety standards, requiring manufacturers to continuously monitor the performance and dangers connected with their gadgets. This requirement for surveillance systems ensures early detection of adverse occurrences, hence lowering patient risk. Furthermore, advances in digital health technology have enhanced tracking and reporting, making it easier to maintain device security.
Development of AI and Machine Learning Technologies for Improved Vigilance Offer Significant Medical Devices Vigilance Market Opportunity
The advancement of AI and machine learning technology opens up considerable prospects for the medical device vigilance market by increasing the efficiency and accuracy of monitoring and reporting adverse occurrences. These technologies enable automated analysis of large datasets, identifying possible threats faster and more precisely than traditional methods. AI-powered systems can anticipate device faults and safety hazards, lowering reaction time and increasing patient outcomes. Additionally, machine learning models are always improving, adjusting to new data and changing regulatory needs. This invention not only speeds vigilance operations but also improves cost efficiency, making it an important growth area in the medical device sector.
Medical Devices Vigilance Market Segmentation
The global market for medical devices vigilance has been segmented into delivery mode, application, and end-user, and region.
- Delivery mode is classified into on-demand, and on-premises
- Application are divided into therapeutic, diagnostic, surgical, research, and others
- End-user are categorized into business process outsourcing [BPO], clinical research organizations [CROs], original equipment manufacturers [OEMs], and others
- The medical devices vigilance market is geographically split into Europe, North America, Latin America, APAC, and the Middle East and Africa
Medical Devices Vigilance Market Regional Outlook
In terms of medical devices vigilance market analysis, the market in North America is increase rapidly due to the increasing number of medical device adverse events recorded in the United States. For instance, the deadline for reporting adverse events for medical devices under emergency use authorization (EUA) is May 12, 2023. The COVID-19 public health emergency (PHE) issued under Section 319 of the Public Health Service (PHS) Act expired on May 11, 2023. This aspect is projected to promote acceptability of medical device vigilance systems, driving the growth of the medical device vigilance market. Furthermore, the growing trend among manufacturers to outsource vigilance activities while keeping core skills would help drive market expansion.
The increasing adoption of medical device vigilance software by BPO firms will fuel the expansion of the medical device vigilance market. Furthermore, key manufacturers in the medical device vigilance market are focusing on a continuous product innovation strategy with the goal of expanding their product portfolio to compete with other established players and strengthening their foothold in the global medical device vigilance market.
Medical Devices Vigilance Market Players
Medical devices vigilance companies profiled in the report include MDI Consultants, Numerix, Oracle, INTEL, Omnify Software, AssurX, AB-Cube, Sparta Systems, Sarjen Systems, and Xybion.
Enquire Before Buying https://www.acumenresearchandconsulting.com/inquiry-before-buying/1240
Receive our personalized services and customization by clicking here https://www.acumenresearchandconsulting.com/request-customization/1240
Mr. Richard Johnson
Acumen Research and Consulting
India: +91 8983225533


