Herceptin Biosimilar Market Size to Reach USD 23.43 Billion by 2033 growing at 25.7% CAGR - Exclusive Report by Acumen Research and Consulting
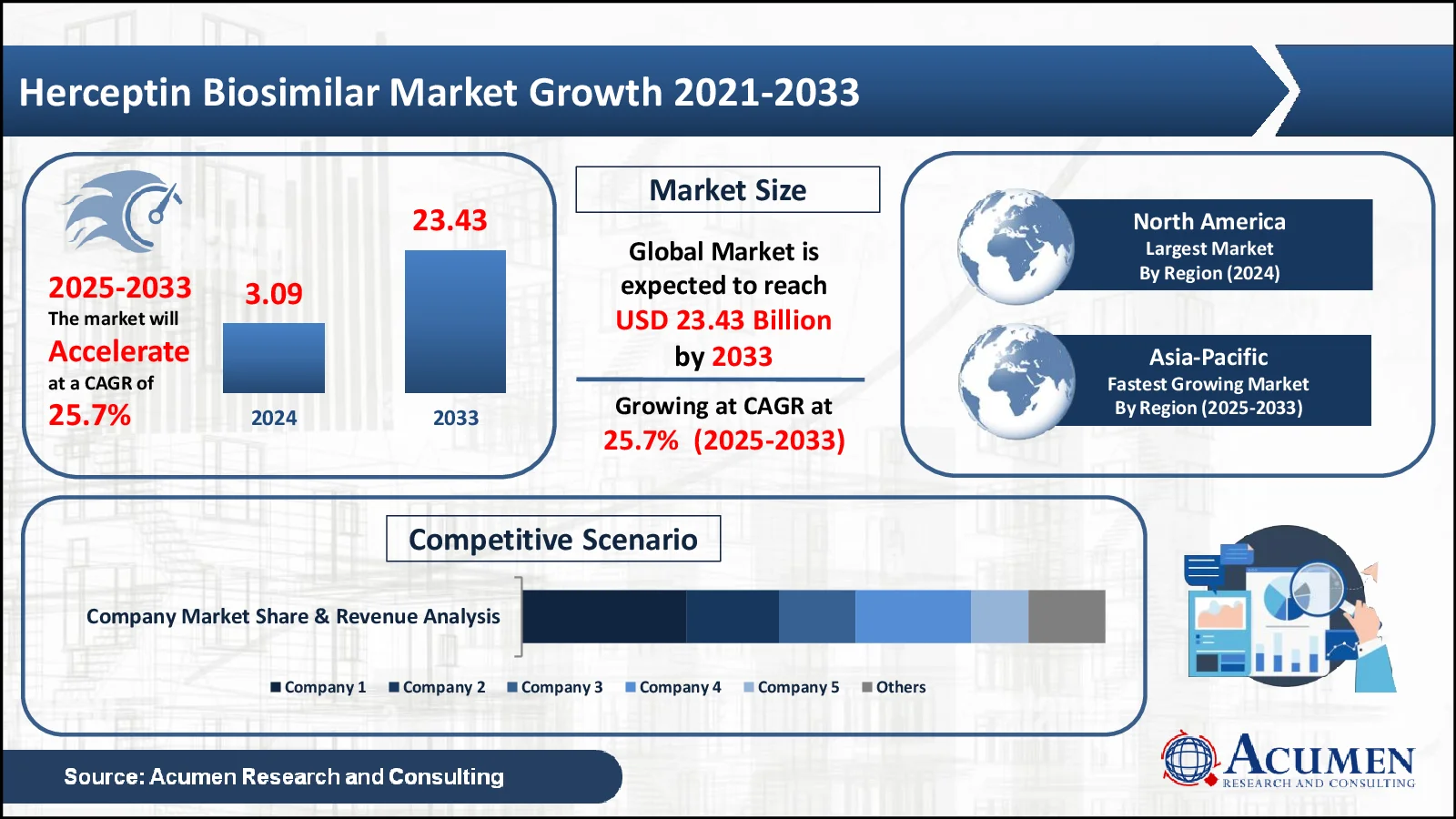
The Herceptin Biosimilar Market Size, valued at USD 3.09 Billion in 2024, is anticipated to surpass USD 23.43 Billion by 2033, reflecting a projected CAGR of 25.7%
Herceptin biosimilars have developed as a more affordable alternative to trastuzumab, a monoclonal antibody used to treat HER2 positive breast and gastric malignancies. These biosimilars deliver the same efficacy and safety as the original biologic while saving money for healthcare systems and patients. The increased prevalence of HER2-positive cancers, together with rising demand for low-cost biologics, has greatly boosted market growth. Regulatory authorizations for biosimilars have prompted pharmaceutical companies to develop and launch herceptin biosimilars in key international markets. As healthcare providers and insurers strive to cut oncology treatment costs, biosimilars are becoming an increasingly essential component of cancer therapy operations.
 Extensive clinical trials demonstrating biosimilars' equivalence to reference biologics help to drive their expanding adoption. Furthermore, rigorous regulatory frameworks established by agencies such as the European Medicines Agency (EMA) and the US Food and Drug Administration ensure that biosimilars meet high-quality standards prior to hitting the market. With several firms joining the market, competition heats up, resulting in price reductions and more accessibility for cancer patients. The herceptin biosimilar market is evolving as biopharmaceutical manufacturing develops and key industry participants collaborate strategically.
Extensive clinical trials demonstrating biosimilars' equivalence to reference biologics help to drive their expanding adoption. Furthermore, rigorous regulatory frameworks established by agencies such as the European Medicines Agency (EMA) and the US Food and Drug Administration ensure that biosimilars meet high-quality standards prior to hitting the market. With several firms joining the market, competition heats up, resulting in price reductions and more accessibility for cancer patients. The herceptin biosimilar market is evolving as biopharmaceutical manufacturing develops and key industry participants collaborate strategically.
Herceptin Biosimilar Market Statistics
- The global market for herceptin biosimilar was worth USD 3.09 billion in 2024.
- The market is expected to grow at a stable annual rate of 25.7% between 2025 and 2033
- North America holds 41% of the herceptin biosimilar industry
- The herceptin biosimilar market is expanding at a 26.3% CAGR in Asia-Pacific
- The hospital pharmacy distribution channel creates the largest income in the market
- The breast cancer application category has considerably contributed to revenue growth
- The herceptin biosimilar industry is driven by strategic acquisitions and mergers by key influencers.
Download Sample Report Copy : https://www.acumenresearchandconsulting.com/request-sample/886
Herceptin Biosimilar Market Dynamics
Growing Cancer Incidence and Cost Containment Efforts Driving Market Growth
The rising incidence of HER2-positive breast and stomach malignancies is a major driver of the herceptin biosimilar market. With rising cancer rates worldwide, healthcare systems confront substantial financial hurdles in providing biologic medicines, which are frequently costly. Herceptin biosimilars present a feasible alternative by providing equivalent therapeutic benefits at a cheaper cost. Governments and healthcare groups are aggressively supporting biosimilars to cut treatment costs and enhance patient access to life-saving pharmaceuticals.
Many countries' policies supporting biosimilar substitution and preferential reimbursement for biosimilars are accelerating market growth. Biosimilars have gained general support among oncologists, and favorable real-world evidence has solidified their place in routine cancer treatment techniques. As more healthcare institutions incorporate biosimilars into their treatment plans, the market is likely to grow gradually in the coming years.
Regulatory Hurdles and Market Competition as Key Challenges
Given the expected development trajectory, regulatory challenges provide a significant barrier to the expansion of the herceptin biosimilar industry. Unlike generic medications, biosimilars necessitate substantial clinical trials to demonstrate similarity to the reference biologic. The regulatory licensing procedure includes extensive analytical, preclinical, and clinical tests to confirm biosimilarity in terms of safety, effectiveness, and immunogenicity. These limitations raise development costs and extend the time to market, limiting new entrants.
Technological Advancements and Strategic Collaborations Creating Market Opportunities
Advances in biopharmaceutical production and novel drug development methodologies are creating new opportunities for herceptin biosimilars. The utilization of contemporary cell culture and purification techniques has enhanced biosimilar production efficiency, leading in higher quality and reduced costs. Companies are leveraging cutting-edge technologies to streamline biosimilar development, resulting in speedier regulatory approval and commercial launch.
Strategic collaborations between pharmaceutical corporations, contract manufacturing organizations (CMOs), and academic institutions are speeding up biosimilar development. Licensing agreements, joint ventures, and co-development initiatives enable companies to pool resources, reduce R&D expenses, and hasten commercialization. Furthermore, expanding biosimilar usage in emerging markets offers enormous development opportunities, as governments in these countries actively support low-cost cancer therapies. As technology advances, the herceptin biosimilar market is likely to grow dramatically in the future years.
Herceptin Biosimilar Market Segmentation
The global herceptin biosimilar market has segmented into 4 categories: application, end-user, distribution channel, and region.
- Application: breast cancer, gastric cancer, and other
- End-User: hospital & clinics, oncology centers, and others
- Distribution Channel: hospital pharmacies, retail pharmacies, and online pharmacies
- Regionally: Asia-Pacific, North America, Europe, Middle East and Africa, and Latin America
Herceptin Biosimilar Market Regional Outlook
The United States has a significant market for herceptin biosimilar, due to a well-established regulatory framework, strong biosimilar adoption policies, and a high frequency of HER2-positive malignancy. Favorable reimbursement standards and a need for cost-effective treatment options have contributed to market growth. Furthermore, large pharmaceutical companies are developing biosimilars at a quick pace, increasing competition and improving patient access.
The Asia-Pacific herceptin biosimilar market is rapidly expanding as cancer prevalence and healthcare investment increase. China, India, and South Korea have emerged as major biosimilar production and export hubs. Government efforts to expand healthcare coverage and reduce treatment costs are hastening business growth. Furthermore, local pharmaceutical companies are quickly developing biosimilars to make them more affordable and accessible in the region.
Herceptin Biosimilar Market Players
Herceptin biosimilar companies profiled in the report include Biocon Limited, Amgen Inc., Merck & Co., Inc., Gedeon Richter Plc, Roche Holding AG, Mabion SA, AryoGen Biopharma, Pfizer Inc., Genor Biopharma Company Ltd, Accord Healthcare Ltd, Mylan N.V, and Samsungbioepis Co., Ltd.
Buy Now This Report : https://www.acumenresearchandconsulting.com/buy-now/0/886
Herceptin Biosimilar Market Insights
|
Parameter |
Details |
|
Size in 2024 |
USD 3.09 Billion |
|
Forecast by 2033 |
USD 23.43 Billion |
|
CAGR During 2025 - 2033 |
25.7% |
|
Largest End-User Segment (% Share 2024) |
Hospital & Clinics – 40% |
|
Largest Region Size (2024) |
North America - USD 1.3 Billion |
|
Fastest Growing Region (% CAGR) |
Asia-Pacific – 26.3% |
|
Key Players Covered |
Biocon Limited, Amgen Inc., Merck & Co., Inc., Gedeon Richter Plc, Roche Holding AG, Mabion SA, AryoGen Biopharma, Pfizer Inc., Genor Biopharma Company Ltd, Accord Healthcare Ltd, Mylan N.V, and Samsungbioepis Co., Ltd. |
|
Request Customization |
Mr. Richard Johnson
Acumen Research and Consulting
India: +91 8983225533
E-mail: [email protected]


