Fabry Disease Treatment Market Size to Reach USD 3.2 Billion by 2032 growing at 7.9% CAGR - Exclusive Report by Acumen Research and Consulting
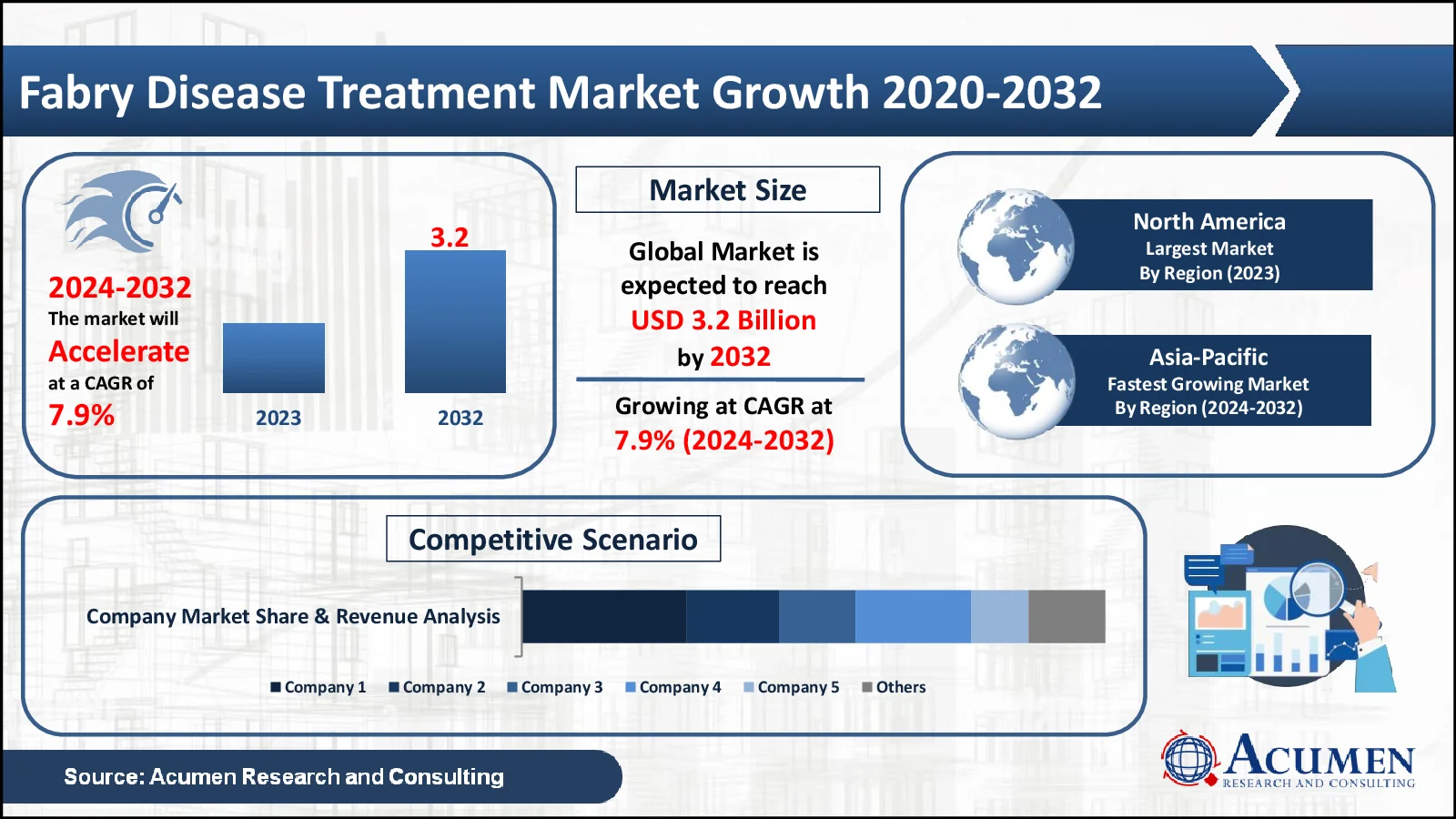
The Fabry Disease Treatment market, valued at USD 1.7 Billion in 2023, is projected to surpass USD 3.2 Billion by 2032, indicating a robust CAGR of 7.9%
Fabry disease is a rare inherited lysosomal storage disorder caused by a genetic mutation that impairs the function of the alpha-galactosidase enzyme. The disease often manifests with late-onset, mild symptoms, leading to many cases going undiagnosed. Enzyme replacement therapy (ERT) is the standard treatment for managing fabry disease. In the EU, Sanofi's Fabrazyme and Shire's Replagal are the only approved ERTs, while in the U.S., only Fabrazyme has received approval. The market is experiencing significant growth due to the increasing number of fabry disease cases, the expanding use of novel treatments like chaperone therapy, and the potential approval of pipeline drugs, including substrate reduction therapies and new enzyme replacement therapies.
However, regulatory challenges and lengthy approval processes became a restraint for the fabry disease treatment market. Furthermore, growth potential in emerging markets with improving healthcare infrastructure prompted the fabry disease treatment market. For instance, May 2023: Sangamo Therapeutics, Inc., a genomic medicine company, received Fast Track Designation from the FDA for isaralgagene civaparvovec, or ST-920, a wholly owned gene therapy product candidate for the treatment of Fabry disease. ST-920 is currently being evaluated in the Phase 1/2 STAAR study, with a total of 20 patients dosed to date.
Fabry Disease Treatment Market Statistics
- The global fabry disease treatment market, generating USD 1.7 billion in 2023, projects a robust CAGR exceeding 7.9% from 2024 to 2032
- North America leads with a substantial revenue USD 561 million in 2023
- Asia-Pacific anticipates impressive growth with a projected CAGR of 9%
- Based on treatment, Enzyme Replacement Therapy (ERT) dominance, holds 65% of market share in 2023
- Based on route of administration, intravenous route sub-segment accomplished notable share in 2023
- A discernible trend in the fabry disease treatment market is shift towards personalized and precision medicine approaches
Access Table Of Content: https://www.acumenresearchandconsulting.com/table-of-content/fabry-disease-treatment-market
Fabry Disease Treatment Market Dynamics
Increasing Awareness and Diagnosis Rates of Rare Diseases like Fabry Fuels the Fabry Disease Treatment Market Value
Increasing awareness and improved diagnostic technologies are significantly boosting the Fabry disease treatment market. As more healthcare professionals become familiar with rare conditions like Fabry disease, early and accurate diagnoses are on the rise. This heightened awareness leads to increased patient identification and, consequently, a greater demand for treatment options. Advances in diagnostic methods also enable earlier intervention, which can improve treatment outcomes and drive market growth. Additionally, growing public awareness campaigns and patient advocacy contribute to a higher prevalence of reported cases. All these factors combine to fuel the expansion of the Fabry disease treatment market.
Expansion of Treatment Options with Innovative Gene and Enzyme Replacement Therapies Offer Significant Fabry Disease Treatment Market Opportunity
The fabry disease treatment market is experiencing a surge of opportunity due to the expansion of innovative gene and enzyme replacement therapies. These advanced treatments address the underlying genetic defect causing the disease by either directly correcting the gene or supplementing the deficient enzyme. Gene therapies aim to provide a long-lasting solution by delivering functional copies of the gene responsible for producing the enzyme. Enzyme replacement therapies, on the other hand, involve regular infusions of the missing enzyme to manage symptoms and prevent disease progression. The growth in these therapeutic options not only enhances patient outcomes but also opens new avenues for market expansion and investment. This shift represents a significant advancement in the management of fabry disease, offering hope for improved quality of life and disease management.
Fabry Disease Treatment Market Segmentation
The global market for fabry disease treatment has been segmented into treatment, route of administration, and distribution channel, and region.
- Treatment is classified into enzyme replacement therapy (ERT), chaperone treatment, substrate reduction therapy (SRT), and others
- Route of administration are divided into intravenous route and oral route
- Distribution channel are categorized into hospital pharmacy, retail pharmacy, and online pharmacy
- The fabry disease treatment market is geographically split into Europe, North America, Latin America, APAC, and the Middle East and Africa
Fabry Disease Treatment Market Regional Outlook
In terms of fabry disease treatment market analysis, North America led the regional market, followed by Europe. The region's growth was driven by the adoption of novel treatments, superior healthcare facilities, and favorable reimbursement policies. The inclusion of high-cost medications, such as fabrazyme, in health insurance programs and supportive healthcare legislation are encouraging pharmaceutical companies to increase their R&D efforts in rare diseases. For instance, in May 2023, the FDA approved Elfabrio (pegunigalsidase alfa-iwxj) for treating adult patients with Fabry disease. This approval came from Chiesi Global Rare Diseases and Protalix BioTherapeutics, Inc. Elfabrio is provided as a preservative-free solution in single-dose vials, each containing 20mg/10mL of pegunigalsidase alfa-iwxj. The treatment is administered via intravenous infusion every two weeks.
The Asia-Pacific region offers significant growth opportunities for pharmaceutical companies due to rising healthcare spending and improving infrastructure. It is expected to experience the fastest growth in the coming years, followed by Latin America, largely due to the large populations in emerging countries.
Fabry Disease Treatment Market Players
Fabry disease treatment companies profiled in the report include ISU Abxis Co Ltd, Green Cross Pharma Pte Ltd., Protalix Biotherapeutics Inc., Amicus Therapeutics Inc., Sanofi S.A., JCR Pharmaceuticals Co Ltd., Moderna Therapeutics Inc., Idorsia Pharmaceuticals Ltd, and Takeda Pharmaceutical Company Limited.
Enquire Before Buying https://www.acumenresearchandconsulting.com/inquiry-before-buying/1066
Receive our personalized services and customization by clicking here https://www.acumenresearchandconsulting.com/request-customization/1066
Mr. Richard Johnson
Acumen Research and Consulting
India: +91 8983225533


