Embolic Protection Devices Market Size to Reach USD 1,234.1 Million by 2032 growing at 7.9% CAGR - Exclusive Report by Acumen Research and Consulting
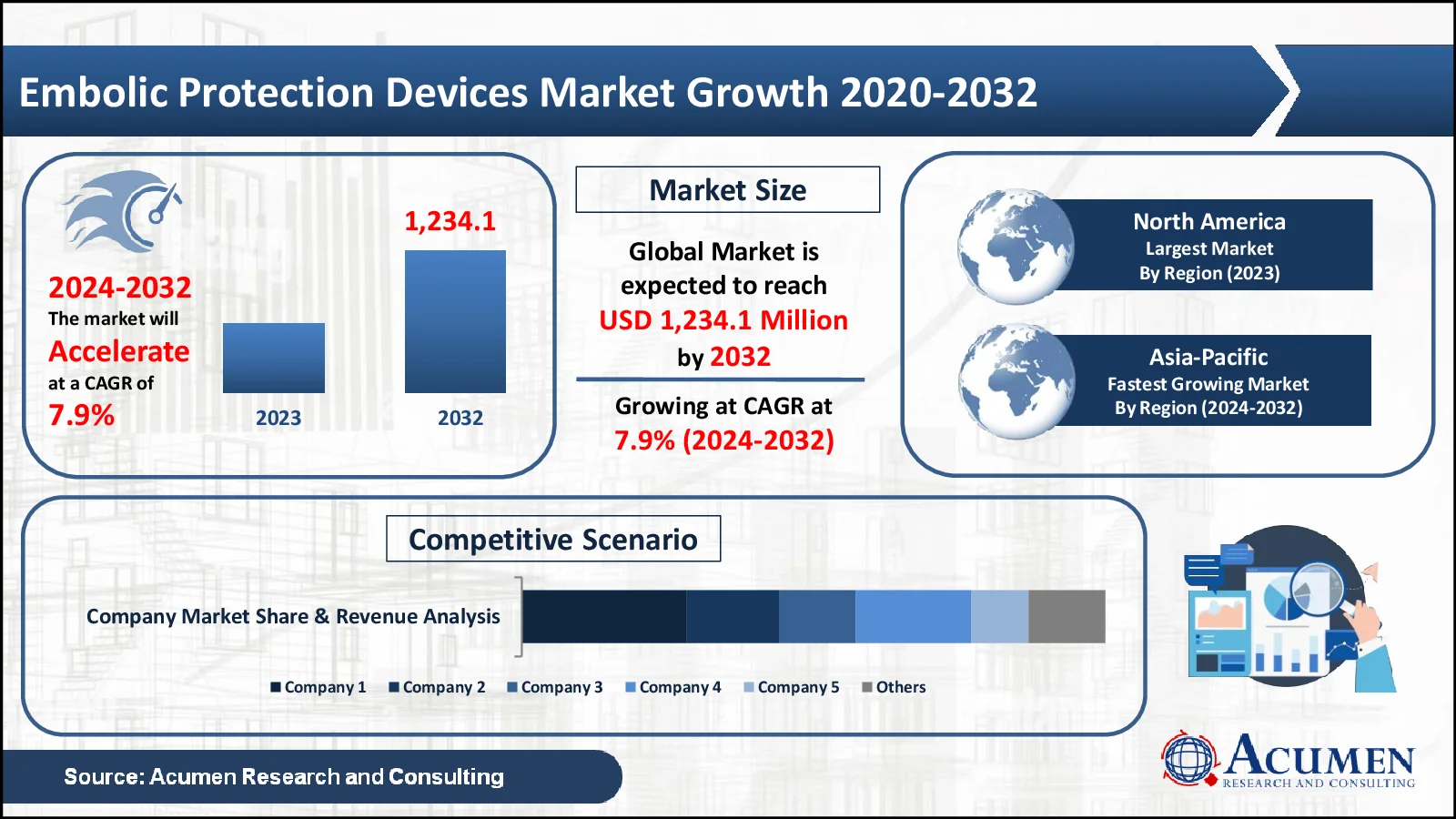
The Embolic Protection Devices market, valued at USD 627.8 Million in 2023, is projected to surpass USD 1,234.1 Million by 2032, indicating a robust CAGR of 7.9%.
Embolic protection devices (EPDs) are advanced medical instruments designed to prevent emboli-small clots or debris-from traveling and causing blockages in other parts of the bloodstream during cardiovascular procedures. These devices are vital in procedures such as carotid artery stenting and transcatheter aortic valve replacement, where the risk of embolic events is high. EPDs work by capturing or filtering embolic material before it can enter the bloodstream, thus reducing the risk of complications like stroke or myocardial infarction. They come in various types, including filter-based devices that trap debris and occlusion-based devices that temporarily block blood flow to manage emboli. The design and technology of EPDs are continually advancing, improving their effectiveness and safety. These innovations enhance the performance of EPDs, leading to better patient outcomes and reduced risk of adverse events during complex cardiovascular procedures.
The embolic protection devices market faced constraints due to stringent regulatory approvals and standards. However, the expansion of procedural applications and indications has driven growth in this market.
Embolic Protection Devices Market Statistics
- The worldwide embolic protection devices market is expected to generate USD 627.8 million in 2023, with a CAGR of 7.9% from 2024 to 2032
- North America leads with a revenue of USD 213.4 million in 2023
- Asia-Pacific is expected to rise at an amazing CAGR of 8.6%
- Distal occlusion filters dominate the market with 63% share in 2023
- Cardiovascular diseases sub-segment gathered 39% share
- Hospitals among end-user have 51% share in 2023
- Technological advancements are resulting in more effective and user-friendly embolic protection devices
Access Table Of Content: https://www.acumenresearchandconsulting.com/table-of-content/embolic-protection-devices-market
Embolic Protection Devices Market Dynamics
Growing Awareness and Adoption of Minimally Invasive Procedures Fuels the Embolic Protection Devices Market Value
The rise in awareness and preference for minimally invasive procedures is significantly boosting the market value for embolic protection devices. For instance, according to American Academy of Facial Plastic and Reconstructive Surgery, the new data indicates that in 2023, 83 percent of all procedures were minimally invasive in America. These devices, essential in safeguarding patients from potential complications during such procedures, are becoming increasingly integral as healthcare providers adopt less invasive techniques. The growing emphasis on patient safety and reduced recovery times is driving demand for these protective tools. Additionally, advancements in technology are enhancing the effectiveness and appeal of embolic protection devices. As a result, the market is experiencing accelerated growth, reflecting the broader shift towards minimally invasive medical interventions.
Rising Demand in Emerging Markets Offer Significant Embolic Protection Devices Market Opportunity
Rising demand in emerging markets presents a substantial opportunity for the embolic protection devices market due to increasing awareness and access to advanced healthcare. As these regions experience economic growth, there is a corresponding rise in the prevalence of cardiovascular diseases, driving the need for effective embolic protection. Improved healthcare infrastructure and higher disposable incomes contribute to the adoption of these specialized devices. Additionally, expanding medical insurance coverage supports the affordability and availability of advanced treatments. Companies that target these emerging markets can capitalize on this growing demand by tailoring their products to local needs and regulations. This presents a significant growth avenue for stakeholders in the embolic protection sector.
Embolic Protection Devices Market Segmentation
The global market for embolic protection devices has been segmented into product, application, and end-users, and region
- Product is classified into distal occlusion filters, proximal occlusion filters, and distal filters
- Application are divided into neurovascular diseases, cardiovascular diseases, and peripheral vascular diseases
- End-users are categorized into hospitals, ambulatory surgical centers (ASCs), and specialty clinics
- The embolic protection devices market is geographically split into Europe, North America, Latin America, APAC, and the Middle East and Africa
Embolic Protection Devices Market Regional Outlook
Regional analysis of embolic protection devices market, North America leads the market due to its advanced healthcare infrastructure, high adoption rates of new technologies, and significant investments in research and development. The region benefits from a strong presence of major medical device companies and a well-established regulatory framework that supports innovation. For instance, in June 2022, Cook Medical announced that the FDA had approved its NirMesh Vascular Occlusion Device for treating patients with varicose veins.
Conversely, Asia Pacific is the fastest-growing region in this market, driven by rapid economic growth, increasing healthcare expenditures, and rising awareness about advanced medical technologies. For instance, in the 2023-24 fiscal year, the Ministry of Health and Family Welfare has been allocated ₹89,155 crore, marking a 13% increase compared to the revised estimates for 2022-23. The expanding aging population and improving healthcare facilities in countries like China and India contribute to this growth. Additionally, the demand for sophisticated medical devices is escalating as healthcare systems in the region evolve and prioritize preventive measures in cardiovascular care.
Embolic Protection Devices Market Players
Embolic protection devices companies profiled in the report include Abbott Laboratories, Allium Medical Solutions Ltd, Gore Medical, Transverse Medical, Inc., Edward Lifesciences, Thermo Fischer Scientific Inc., Medtronic Plc., Cardinal Health, Boston Scientific Corp, and Siemens Healthcare GmbH.
Enquire Before Buying https://www.acumenresearchandconsulting.com/inquiry-before-buying/982
Receive our personalized services and customization by clicking here https://www.acumenresearchandconsulting.com/request-customization/982
Mr. Richard Johnson
Acumen Research and Consulting
India: +91 8983225533


