Large Bore Vascular Closure Device Market Size to Reach USD 1,827.9 Million by 2032 growing at 7.9% CAGR - Exclusive Report by Acumen Research and Consulting
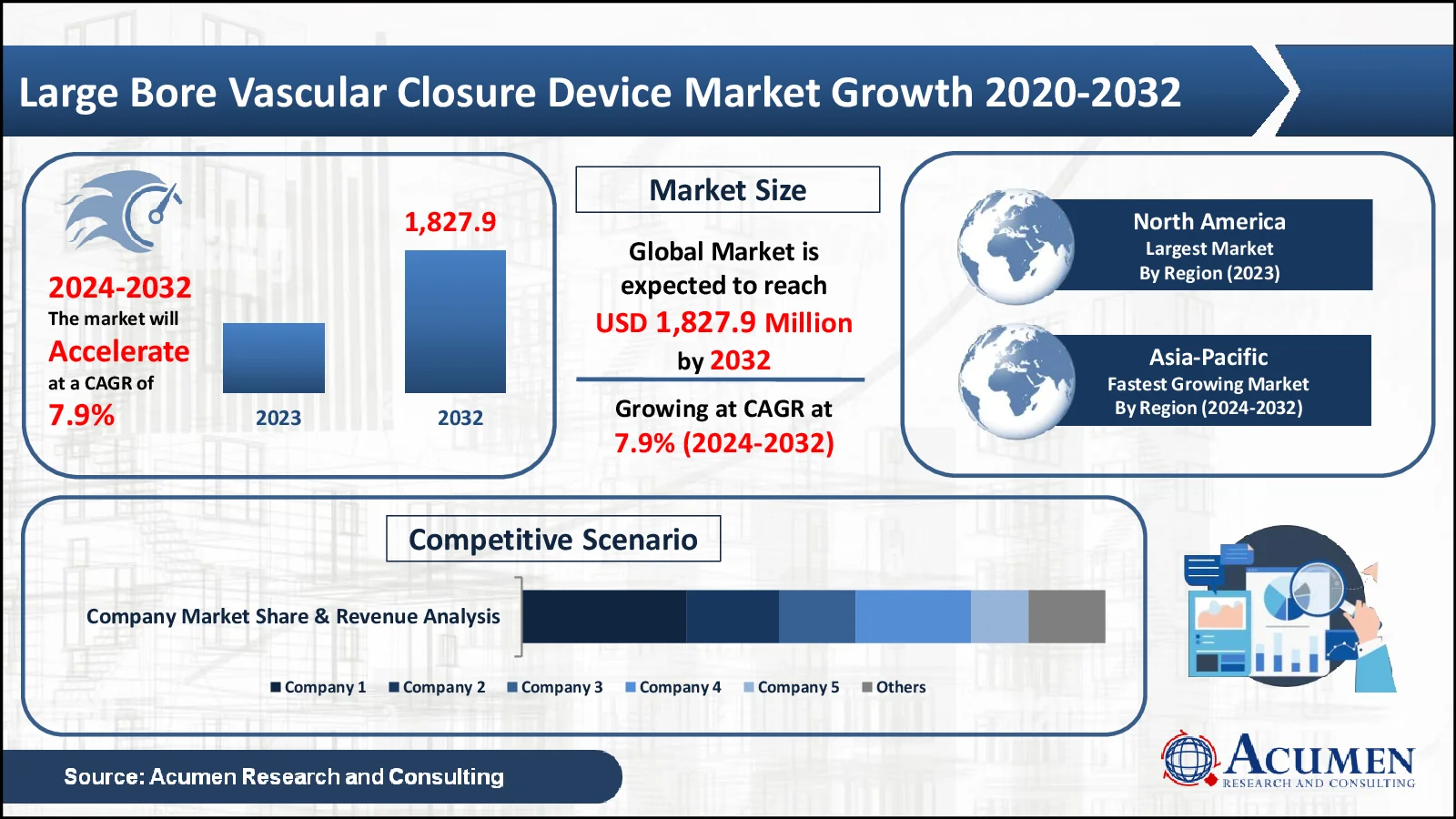
The Large Bore Vascular Closure Device Market, valued at USD 928.3 Million in 2023, is anticipated to surpass USD 1,827.9 Million by 2032, reflecting a projected CAGR of 7.9%
The large bore vascular closure device market is expanding rapidly, driven by an increase in cardiovascular treatments and technical developments. These devices are critical for closing big puncture sites in arteries after catheter-based treatments such as transcatheter aortic valve replacements (TAVR) and endovascular aneurysm repair (EVAR). The rising prevalence of cardiovascular diseases, aging populations, and a preference for minimally invasive surgeries are all driving up demand. Manufacturers are emphasizing on innovation, resulting in the continual release of sophisticated products with improved efficacy, safety, and convenience of use.
However, high gadget costs and tight regulatory guidelines are barriers to commercial expansion. Despite these challenges, the LBVCD market is predicted to expand as healthcare systems worldwide invest in novel vascular technology, aided by rising healthcare costs.
Large Bore Vascular Closure Device Market Statistics
- In 2022, the global large bore vascular closure device market was valued at USD 928.3 million
- The market is expected to grow at a stable annual pace of 7.9% from 2023 to 2032
- The North America area accounts for 37% of the large bore vascular closure device market
- Asia-Pacific is increasing at a CAGR of 8.6% in the large bore vascular closure device market
- The active closure devices product segment generates the maximum revenue
- The femoral arterial application sector has notably contributed to revenue growth in the large bore vascular closure device market
- Rising demand in emerging economies creates new growth avenues opens up new opportunities for participation
Access Table Of Content: https://www.acumenresearchandconsulting.com/table-of-content/large-bore-vascular-closure-device-market
Large Bore Vascular Closure Device Market Dynamics
Rise in Cardiovascular Procedures Fuels the Large Bore Vascular Closure Device Market
The growing number of cardiovascular operations, particularly that requiring large-bore access, such as TAVR and EVAR, is a major driver of the LBVCD industry. The global growth in cardiovascular illnesses, which can be linked to aging populations, sedentary lifestyles, and poor diets, has greatly raised demand for catheter-based therapies. These procedures are less intrusive than typical surgeries, making them appealing to both patients and healthcare practitioners.
The increase in such operations has directly resulted in a surge in demand for effective closure devices capable of handling larger puncture sites. Large bore vascular closure devices are critical in these procedures to reduce problems like bleeding, improve recovery time, and shorten hospital stays. This rising patient base, as well as the worldwide healthcare system’s transition to minimally invasive treatments, is important drivers of the market.
High Device Costs and Regulatory Challenges May Impede the Market
The high cost of large bore vascular closure devices, along with tight regulatory regulations, places a severe constraint on the market. These devices are made with modern materials and complex technology, making them costly to manufacture. The expensive cost of such devices hinders adoption for healthcare practitioners, particularly in poor countries, where budget constraints and reimbursement policies may not cover the entire cost.
Furthermore, the regulatory licensing process for medical devices, especially in North America and Europe, is demanding. These tight regulatory frameworks safeguard patient safety while also delaying the entrance of new products to the market. Manufacturers must traverse rigorous approval procedures that include clinical research, adherence to safety standards, and detailed paperwork. This not only extends the time to market, but also raises development expenditures. As a result, the high cost of innovation and regulatory delays impose restrictions on smaller producers and prevent widespread adoption in cost-sensitive sectors.
Technological Advancements Offers Significant Large Bore Vascular Closure Device Market Opportunities
Technological improvements in large bore vascular closure devices create a considerable possibility for market expansion. Innovations aiming at improving patient outcomes, lowering procedure complications, and increasing device efficacy are likely to drive market acceptance. Recent advancements include devices that provide faster deployment, more precision, and better hemostasis, minimizing the need for post-procedure manual compression. The incorporation of modern imaging technology has also improved closure devices' capacity to seal puncture wounds with greater precision, lowering the risk of bleeding and other problems.
In addition, the development of bioresorbable materials is gaining traction, enabling for closure devices that disintegrate over time, eliminating the need for device retrieval and lowering long-term risks. These innovations not only increase patient safety, but also reduce hospital stays and healthcare costs, making them desirable to both patients and providers. As prominent market participants engage extensively in R&D, the launch of innovative technologies is projected to open up new growth prospects, especially in regions with advanced healthcare infrastructures.
Large Bore Vascular Closure Device Market Segmentation
The worldwide large bore vascular closure device market is divides into 2 segments: product applications, and regional markets
- Product: passive closure devices, and active closure devices
- Application: femoral arterial, and transradial arterial
- Regional: Latin America, Asia-Pacific, North America, the Middle East & Africa, and Europe
Large Bore Vascular Closure Device Market Regional Outlook
North America leads the large bore vascular closure device market, owing to a high prevalence of cardiovascular illnesses, a well-established healthcare infrastructure, and major investments in medical device research and development. The United States, in particular, is in the vanguard due to a large presence of leading device makers, significant healthcare expenditures, and a growing senior population predisposed to cardiovascular disease. The region's emphasis on minimally invasive procedures drives up demand for LBVCDs.
The Asia-Pacific region promises a substantial development opportunity as the burden of cardiovascular disease rises and growing economies such as China and India improve their healthcare systems. Increased healthcare costs and awareness of minimally invasive surgeries are driving up demand for LBVCDs in the region. However, the exorbitant cost of these gadgets may limit their use in certain nations. Government activities focused at enhancing healthcare accessibility are anticipated to offset this issue, resulting in future market growth.
Large Bore Vascular Closure Device Market Players
Large bore vascular closure device companies profiled in the report include Cardinal Health, Inseal Medical, Medeon Biodesign, Vascular Solutions, Vasorum, Transluminal Technologies, Abbott Vascular, Morrris Innovative, Essential Medical, and St. Jude Medical.
Enquire Before Buying https://www.acumenresearchandconsulting.com/inquiry-before-buying/1274
Receive our personalized services and customization by clicking here https://www.acumenresearchandconsulting.com/request-customization/1274
Mr. Richard Johnson
Acumen Research and Consulting
India: +91 8983225533


