eClinical Solutions Market Size to Reach USD 27.1 Billion by 2032
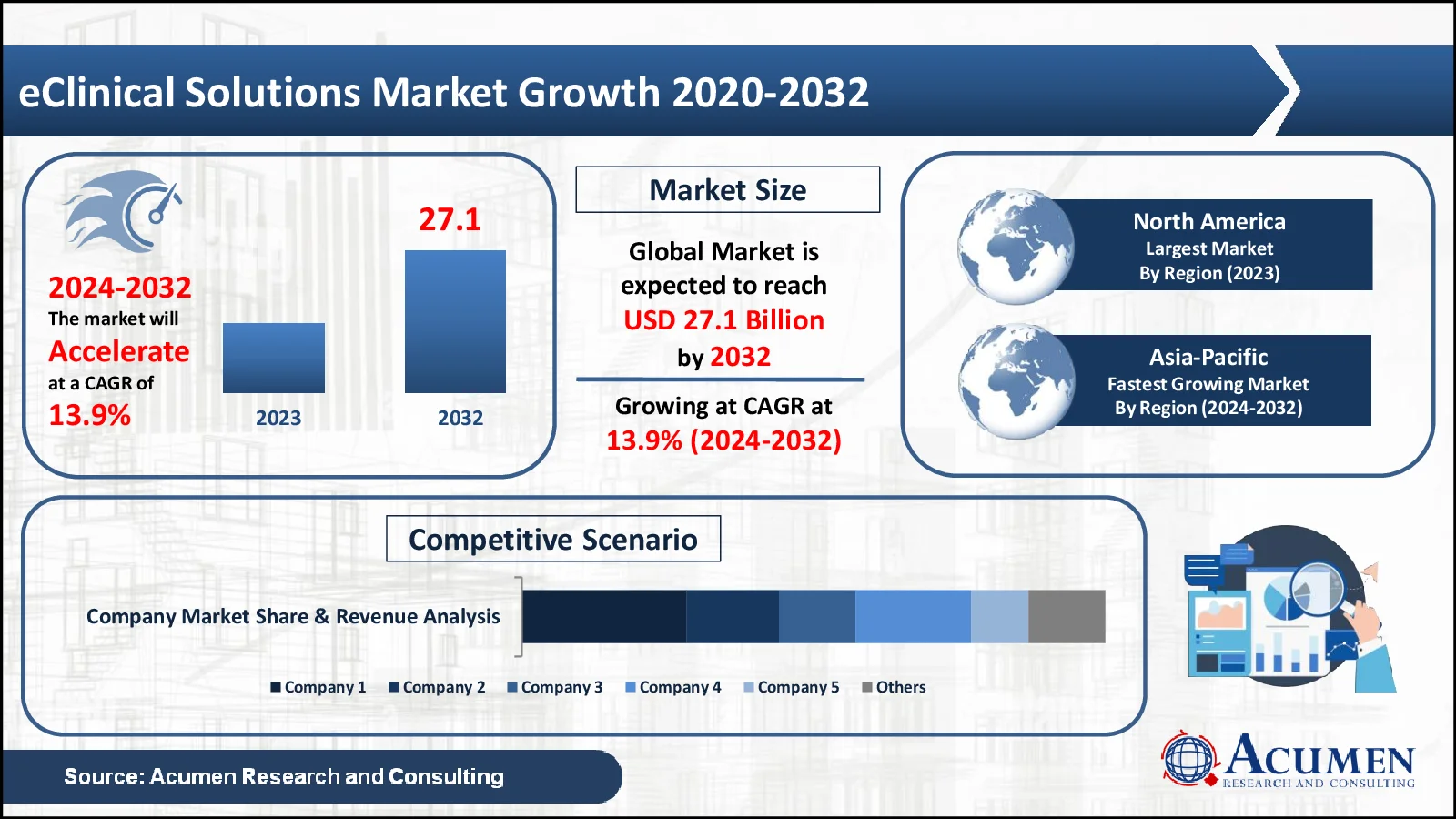
The eClinical Solutions Market, valued at USD 8.5 Billion in 2023, is anticipated to surpass USD 27.1 Billion by 2032, reflecting a projected CAGR of 13.9%
The eClinical solutions industry is transforming the healthcare and clinical research industries by combining cutting-edge technology to speed clinical trials and improve data management. These solutions use cloud computing, artificial intelligence (AI), and analytics to increase operational efficiency, lower costs, and assure regulatory compliance. The market is predicted to expand significantly as clinical trials get more complicated, clinical data volumes increase, and a greater emphasis is placed on patient-centric approaches.
EClinical Solutions Market Statistics
- The global eClinical solutions market was valued at USD 8.5 billion in 2023
- The market is projected to increase at a stable annual pace of 13.9% from 2024-2032
- North America accounts for 49% of the eClinical solutions market
- Asia-Pacific is growing at a CAGR of 14.8% within the eClinical solutions market
- The cloud and web based delivery mode has the largest earning eClinical solutions market
- The eClinical solutions market has seen significant revenue growth from phase III clinical trials
- Rising outsourcing of clinical trials to CROs is a trend in the eClinical solutions market
Download Sample Report Copy From Here: https://www.acumenresearchandconsulting.com/request-sample/1637
EClinical Solutions Market Dynamics
Rising Complexity of Clinical Trials Drives eClinical Solutions Market Growth
The pharmaceutical and biotechnology sectors are seeing an increase in the complexity of clinical trials as personalized medicine and targeted therapeutics become more prevalent. These improvements demand strong systems for managing various data kinds, such as genomics and real-world evidence. eClinical solutions offer integrated platforms for real-time monitoring, fast data collection, and streamlined analysis. The increased demand for efficient trial management has accelerated the adoption of eClinical solutions throughout the healthcare sector.
Regulatory Support and Industry Standards
Regulatory bodies, such as the FDA and EMA, are increasingly promoting the use of advanced data management systems to assure clinical trial integrity and openness. Initiatives such as the Clinical Trials Transformation Initiative (CTTI) promote the digitalization of clinical trials, which accelerates the adoption of eClinical technologies. These regulations, when paired with industry standards for interoperability and data interchange, create a conducive climate for business expansion.
eClinical Solutions Market Restraints
High Implementation and Maintenance Costs
Despite the long-term benefits, the high costs of developing and maintaining eClinical systems are a considerable hurdle, particularly for small and medium-sized businesses (SMEs). Expenses for software license, infrastructure changes, and staff training might inhibit uptake, especially in resource-constrained environments.
Data Integration and Interoperability Issues
The absence of defined protocols for data integration and interoperability is a significant hurdle. Clinical trials frequently involve several stakeholders, each using their own systems and data formats. The ensuing fragmentation can cause inefficiencies and errors, limiting the full potential of eClinical solutions. Efforts to solve these difficulties necessitate major expenditure in defining and implementing universal standards.
Opportunities in eClinical Solutions Market
Emergence of Decentralized Clinical Trials
Decentralized clinical trials (DCTs) are gaining popularity as a patient-centered strategy to doing research. These trials use telemedicine, wearable technology, and remote monitoring to reduce patient burden and increase participation. eClinical solutions complement DCTs by enabling seamless data capture, remote access, and real-time analysis. The increasing adoption of DCTs creates considerable opportunity for market players to broaden their offers.
Integration of Blockchain Technology
Blockchain technology is developing as a game changer in clinical research, providing unprecedented data security, transparency, and traceability. Integrating blockchain into eClinical solutions allows stakeholders to maintain trial data integrity, reduce the danger of data tampering, and increase patient trust. This technology also enables effective data sharing and collaboration, opening up new avenues for market expansion.
Increased Focus on Patient Engagement
As the industry transitions to a more patient-centered approach, there is a greater emphasis on solutions that improve patient engagement and retention. eClinical solutions with user-friendly interfaces, mobile applications, and personalized communication capabilities can greatly improve patient experiences. This approach corresponds with broader healthcare trends, opening up new opportunities for market innovation and growth.
EClinical Solutions Market Segmentation
The worldwide eclinical solutions market is divided into 5 segments: product, delivery mode, clinical trial phase, end-use, and regional markets
- Product: electronic data capture and clinical data management systems, clinical trial management systems, clinical analytics platforms, randomization and trial supply management, clinical data integration platforms, electronic clinical outcome assessment solutions, safety solutions, electronic trial master file systems, regulatory information management solutions, and others
- Delivery Mode: on-premise, and cloud and web based
- Clinical Trial Phase: phase I clinical trials, phase II clinical trials, phase III clinical trials, and phase IV clinical trials
- End-use: pharmaceutical and biopharmaceutical companies, contract research organizations, consulting service companies, medical device manufacturers, hospitals, and academic research institutes
- Regional: the Middle East & Africa, Asia-Pacific, Europe, Latin America, and North America
EClinical Solutions Market Regional Outlook
North America dominates the eClinical solutions market, owing to its advanced healthcare infrastructure, strong regulatory framework, and large R&D investments. The United States, in particular, is a leader in the adoption of cutting-edge technologies, with major stakeholders actively working together to produce creative solutions. The region's emphasis on individualized treatment and decentralized trials accelerates market expansion. Furthermore, the presence of major pharmaceutical corporations and clinical research organizations (CROs) ensures a steady demand for eClinical solutions.
eClinical Solutions Market Players
eClinical solutions companies profiled in the report include Bio-optronics, Medsharing, Dassault Systemes (Medidata), IBM, OpenClinica, Anju Software (OmniComm System), Multihealth Group (Clinfile), Oracle Corporation, Ennov, Clario, and Datatrak.
Buy Now This Report: https://www.acumenresearchandconsulting.com/buy-now/0/1637
|
Parameter |
Details |
|
Size in 2023 |
USD 8.5 Billion |
|
Forecast by 2032 |
USD 27.1 Billion |
|
CAGR During 2024 - 2032 |
13.9% |
|
Largest Region Size (2023) |
North America- USD 4.2 Billion |
|
Fastest Growing Region (% CAGR) |
Asia-Pacific – 14.8% |
|
Key Players Covered |
Medsharing, Bio-optronics, Dassault Systemes (Medidata), OpenClinica, IBM, Anju Software (OmniComm System), Oracle Corporation, Multihealth Group (Clinfile), Clario, Ennov, and Datatrak. |
|
Click Here To Customize Report |
Mr. Richard Johnson
Acumen Research and Consulting
India: +91 8983225533
E-mail: [email protected]


